Red Seaweeds Sarcodiotheca gaudichaudii and Chondrus crispus down Regulate Virulence Factors of Salmonella Enteritidis and Induce Immune Responses in Caenorhabditis elegans
- PMID: 27065981
- PMCID: PMC4814495
- DOI: 10.3389/fmicb.2016.00421
Red Seaweeds Sarcodiotheca gaudichaudii and Chondrus crispus down Regulate Virulence Factors of Salmonella Enteritidis and Induce Immune Responses in Caenorhabditis elegans
Abstract
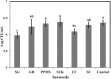
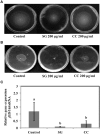
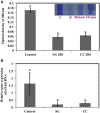
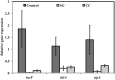
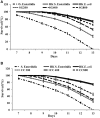
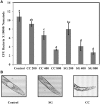
Red seaweeds are a rich source of unique bioactive compounds and secondary metabolites that are known to improve human and animal health. S. Enteritidis is a broad range host pathogen, which contaminates chicken and poultry products that end into the human food chain. Worldwide, Salmonella outbreaks have become an important economic and public health concern. Moreover, the development of resistance in Salmonella serovars toward multiple drugs highlights the need for alternative control strategies. This study evaluated the antimicrobial property of red seaweeds extracts against Salmonella Enteritidis using the Caenorhabditis elegans infection model. Six red seaweed species were tested for their antimicrobial activity against S. Enteritidis and two, Sarcodiotheca gaudichaudii (SG) and Chondrus crispus (CC), were found to exhibit such properties. Spread plate assay revealed that SG and CC (1%, w/v) significantly reduced the growth of S. Enteritidis. Seaweed water extracts (SWE) of SG and CC, at concentrations from 0.4 to 2 mg/ml, significantly reduced the growth of S. Enteritidis (log CFU 4.5-5.3 and log 5.7-6.0, respectively). However, methanolic extracts of CC and SG did not affect the growth of S. Enteritidis. Addition of SWE (0.2 mg/ml, CC and SG) significantly decreased biofilm formation and reduced the motility of S. Enteritidis. Quantitative real-time PCR analyses showed that SWE (CC and SG) suppressed the expression of quorum sensing gene sdiA and of Salmonella Pathogenesis Island-1 (SPI-1) associated genes sipA and invF, indicating that SWE might reduce the invasion of S. Enteritidis in the host by attenuating virulence factors. Furthermore, CC and SG water extracts significantly improved the survival of infected C. elegans by impairing the ability of S. Enteritidis to colonize the digestive tract of the nematode and by enhancing the expression of C. elegans immune responsive genes. As the innate immune response pathways of C. elegans and mammals show a high degree of conservation, these results suggest that these SWE may also impart beneficial effects on animal and human health.
Keywords: Caenorhabditis elegans; Chondrus crispus; Salmonella enteritidis; Sarcodiotheca gaudichaudii; immune response; virulence factors.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

