Peramivir: A Novel Intravenous Neuraminidase Inhibitor for Treatment of Acute Influenza Infections
- PMID: 27065996
- PMCID: PMC4815007
- DOI: 10.3389/fmicb.2016.00450
Peramivir: A Novel Intravenous Neuraminidase Inhibitor for Treatment of Acute Influenza Infections
Abstract
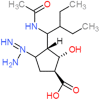
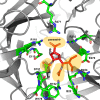
Peramivir is a novel cyclopentane neuraminidase inhibitor of influenza virus. It was approved by the Food and Drug Administration in December 2014 for treatment of acute uncomplicated influenza in patients 18 years and older. For several months prior to approval, the drug was made clinically available under Emergency Use authorization during the 2009 H1N1 influenza pandemic. Peramivir is highly effective against human influenza A and B isolates as well as emerging influenza virus strains with pandemic potential. Clinical trials demonstrated that the drug is well-tolerated in adult and pediatric populations. Adverse events are generally mild to moderate and similar in frequency to patients receiving placebo. Common side effects include gastrointestinal disorders and decreased neutrophil counts but are self-limiting. Peramivir is administered as a single-dose via the intravenous route providing a valuable therapeutic alternative for critically ill patients or those unable to tolerate other administration routes. Successful clinical trials and post-marketing data in pediatric populations in Japan support the safety and efficacy of peramivir in this population where administration of other antivirals might not be feasible.
Keywords: efficacy; influenza; neuraminidase inhibitors; peramivir; safety.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources