Dynamic Protein Acetylation in Plant-Pathogen Interactions
- PMID: 27066055
- PMCID: PMC4811901
- DOI: 10.3389/fpls.2016.00421
Dynamic Protein Acetylation in Plant-Pathogen Interactions
Abstract
Pathogen infection triggers complex molecular perturbations within host cells that results in either resistance or susceptibility. Protein acetylation is an emerging biochemical modification that appears to play central roles during host-pathogen interactions. To date, research in this area has focused on two main themes linking protein acetylation to plant immune signaling. Firstly, it has been established that proper gene expression during defense responses requires modulation of histone acetylation within target gene promoter regions. Second, some pathogens can deliver effector molecules that encode acetyltransferases directly within the host cell to modify acetylation of specific host proteins. Collectively these findings suggest that the acetylation level for a range of host proteins may be modulated to alter the outcome of pathogen infection. This review will focus on summarizing our current understanding of the roles of protein acetylation in plant defense and highlight the utility of proteomics approaches to uncover the complete repertoire of acetylation changes triggered by pathogen infection.
Keywords: acetylation; defense; plant–pathogen interaction; post-translational modification; proteomics.
Figures
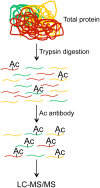
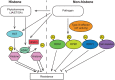
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

