Response-adapted treatment with upfront high-dose chemotherapy followed by autologous stem-cell transplantation rescue or consolidation phase high-dose methotrexate for primary central nervous system lymphoma: a long-term mono-center study
- PMID: 27066340
- PMCID: PMC4786507
- DOI: 10.1186/s40064-016-1954-6
Response-adapted treatment with upfront high-dose chemotherapy followed by autologous stem-cell transplantation rescue or consolidation phase high-dose methotrexate for primary central nervous system lymphoma: a long-term mono-center study
Abstract
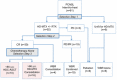
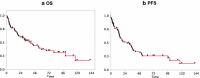
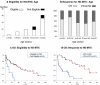
Treatment regimens for primary central nervous system lymphoma (PCNSL) include high-dose methotrexate (HD-MTX)-based chemotherapy, with or without radiotherapy and are based on studies of selected patient groups. This retrospective study assessed a consistent strategy of response-adapted protocol applied for patients including age >65 years in a cancer center for 10 years longitudinally. Case notes were studied of 61 consecutively treated patients with PCNSL histologically diagnosed between 2003 and 2013. Clinical follow-up during and after treatment included neurologic examination and magnetic resonance imaging. Of the patients studied, 14.8 % (9/61) were clinically unfit for chemotherapy; the remaining 85.2 % (52/61) of patients were treated with HD-MTX. Of these patients, 58 % (30/52) achieved an initial complete response, with a median survival of 100.1 months. Of these response-adapted patients, 33 % (10/30) were <65 years and were treated with upfront high-dose chemotherapy and autologous stem-cell transplantation (HDC-ASCT). The remaining response-adapted patients included 53 % (16/30) who were ≥65 years underwent consolidation with HD-MTX, and 14 % (4/30) who chose radiotherapy. The median survival of patients with HDC-ASCT had not yet been reached compared with 67.6 months for patients with HD-MTX consolidation treatment (p = 0.26). At the end of the study, 75 % (39/52) of patients had died mainly owing to progression or relapse of PCNSL. Multivariate analysis showed that age younger than 65 years (p = 0.02) and complete response for up-front HD-MTX (p = 0.001) were independent prognostic indicators of overall survival. In conclusion, this single-center retrospective clinical study has shown that treatment of PCNSL with upfront HDC-ASCT and consolidation phase HD-MTX monotherapy may be feasible, even for elderly patients in a routine clinical setting, using the three-step selection by eligibility and response to initial HD-MTX, and age threshold of 65 years for ASCT.
Keywords: Autologous stem-cell transplant; Chemotherapy; Methotrexate; Primary central nervous system lymphoma; Radiotherapy; Rituximab.
Figures

Similar articles
-
Clinical characteristics and survival outcomes of patients with primary central nervous system lymphoma treated with high-dose methotrexate-based polychemotherapy and consolidation therapies.Eur J Cancer. 2024 Dec;213:115068. doi: 10.1016/j.ejca.2024.115068. Epub 2024 Oct 13. Eur J Cancer. 2024. PMID: 39427440
-
A retrospective study of 222 patients with newly diagnosed primary central nervous system lymphoma-Outcomes indicative for improved survival overtime.Hematol Oncol. 2023 Dec;41(5):838-847. doi: 10.1002/hon.3198. Epub 2023 Jul 5. Hematol Oncol. 2023. PMID: 37403752
-
High-dose chemotherapy and autologous stem cell transplantation for primary central nervous system lymphoma: a multi-centre retrospective analysis from the United Kingdom.Bone Marrow Transplant. 2017 Sep;52(9):1268-1272. doi: 10.1038/bmt.2017.101. Epub 2017 Jun 5. Bone Marrow Transplant. 2017. PMID: 28581466
-
High-dose methotrexate-based regimens and post-remission consolidation for treatment of newly diagnosed primary CNS lymphoma: meta-analysis of clinical trials.Sci Rep. 2021 Jan 22;11(1):2125. doi: 10.1038/s41598-020-80724-0. Sci Rep. 2021. PMID: 33483528 Free PMC article. Review.
-
Haematopoietic stem cell transplantation for treatment of primary CNS lymphoma: single-centre experience and literature review.Eur J Haematol. 2015 Jul;95(1):75-82. doi: 10.1111/ejh.12482. Epub 2015 Mar 23. Eur J Haematol. 2015. PMID: 25546348 Review.
Cited by
-
Stem cell transplantation during cancer.Oncol Lett. 2016 Dec;12(6):4297-4300. doi: 10.3892/ol.2016.5260. Epub 2016 Oct 13. Oncol Lett. 2016. Retraction in: Oncol Lett. 2020 Nov;20(5):222. doi: 10.3892/ol.2020.12085. PMID: 28105145 Free PMC article. Retracted.
-
Extranodal lymphoma: pathogenesis, diagnosis and treatment.Mol Biomed. 2023 Sep 18;4(1):29. doi: 10.1186/s43556-023-00141-3. Mol Biomed. 2023. PMID: 37718386 Free PMC article. Review.
-
Treatment Regimens for Immunocompetent Elderly Patients with Primary Central Nervous System Lymphoma: A Scoping Review.Cancers (Basel). 2021 Aug 24;13(17):4268. doi: 10.3390/cancers13174268. Cancers (Basel). 2021. PMID: 34503078 Free PMC article.
-
Non-Myeloablative Chemotherapy as Consolidation Strategy After High-Dose Methotrexate-Based Chemoimmunotherapy in Patients With Primary CNS Lymphoma: A Retrospective Single Center Study in China.Front Oncol. 2022 Feb 23;12:792274. doi: 10.3389/fonc.2022.792274. eCollection 2022. Front Oncol. 2022. PMID: 35280789 Free PMC article.
-
Diagnosis, prognosis and treatment of primary central nervous system lymphoma in the elderly population (Review).Int J Oncol. 2021 Mar;58(3):371-387. doi: 10.3892/ijo.2021.5180. Epub 2021 Feb 1. Int J Oncol. 2021. PMID: 33650642 Free PMC article. Review.
References
-
- Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol. 1998;16:859–863. - PubMed
-
- Abrey LE, Moskowitz CH, Mason WP, Crump M, Stewart D, Forsyth P, Paleologos N, Correa DD, Anderson ND, Caron D, Zelenetz A, Nimer SD, DeAngelis L. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis. J Clin Oncol. 2003;21:4151–4156. doi: 10.1200/JCO.2003.05.024. - DOI - PubMed
-
- Deckert M, Paulus W. Malignant lymphomas. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO classification of tumours of the central nervous system. Lyon: IARC; 2007. pp. 188–192.
LinkOut - more resources
Full Text Sources
Other Literature Sources

