Cellular Aspects of Shigella Pathogenesis: Focus on the Manipulation of Host Cell Processes
- PMID: 27066460
- PMCID: PMC4814626
- DOI: 10.3389/fcimb.2016.00038
Cellular Aspects of Shigella Pathogenesis: Focus on the Manipulation of Host Cell Processes
Abstract
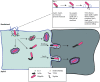
Shigella is a Gram-negative bacterium that is responsible for shigellosis. Over the years, the study of Shigella has provided a greater understanding of how the host responds to bacterial infection, and how bacteria have evolved to effectively counter the host defenses. In this review, we provide an update on some of the most recent advances in our understanding of pivotal processes associated with Shigella infection, including the invasion into host cells, the metabolic changes that occur within the bacterium and the infected cell, cell-to-cell spread mechanisms, autophagy and membrane trafficking, inflammatory signaling and cell death. This recent progress sheds a new light into the mechanisms underlying Shigella pathogenesis, and also more generally provides deeper understanding of the complex interplay between host cells and bacterial pathogens in general.
Keywords: NLR; Nod1 signaling adaptor protein; Nod2 signaling adaptor protein; Shigella; autophagy; bacterial infections; innate immunity; toll-like receptors.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

