Longitudinal Effects of Ketamine on Dendritic Architecture In Vivo in the Mouse Medial Frontal Cortex
- PMID: 27066532
- PMCID: PMC4819286
- DOI: 10.1523/ENEURO.0133-15.2016
Longitudinal Effects of Ketamine on Dendritic Architecture In Vivo in the Mouse Medial Frontal Cortex
Abstract
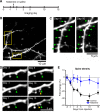
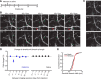
A single subanesthetic dose of ketamine, an NMDA receptor antagonist, leads to fast-acting antidepressant effects. In rodent models, systemic ketamine is associated with higher dendritic spine density in the prefrontal cortex, reflecting structural remodeling that may underlie the behavioral changes. However, turnover of dendritic spines is a dynamic process in vivo, and the longitudinal effects of ketamine on structural plasticity remain unclear. The purpose of the current study is to use subcellular resolution optical imaging to determine the time course of dendritic alterations in vivo following systemic ketamine administration in mice. We used two-photon microscopy to visualize repeatedly the same set of dendritic branches in the mouse medial frontal cortex (MFC) before and after a single injection of ketamine or saline. Compared to controls, ketamine-injected mice had higher dendritic spine density in MFC for up to 2 weeks. This prolonged increase in spine density was driven by an elevated spine formation rate, and not by changes in the spine elimination rate. A fraction of the new spines following ketamine injection was persistent, which is indicative of functional synapses. In a few cases, we also observed retraction of distal apical tuft branches on the day immediately after ketamine administration. These results indicate that following systemic ketamine administration, certain dendritic inputs in MFC are removed immediately, while others are added gradually. These dynamic structural modifications are consistent with a model of ketamine action in which the net effect is a rebalancing of synaptic inputs received by frontal cortical neurons.
Keywords: dendrites; dendritic spines; frontal cortex; ketamine; structural plasticity; two-photon microscopy.
Figures




Similar articles
-
Longitudinal two-photon imaging in somatosensory cortex of behaving mice reveals dendritic spine formation enhancement by subchronic administration of low-dose ketamine.Sci Rep. 2018 Apr 24;8(1):6464. doi: 10.1038/s41598-018-24933-8. Sci Rep. 2018. PMID: 29691465 Free PMC article.
-
Pyramidal Neurons in Different Cortical Layers Exhibit Distinct Dynamics and Plasticity of Apical Dendritic Spines.Front Neural Circuits. 2017 Jun 19;11:43. doi: 10.3389/fncir.2017.00043. eCollection 2017. Front Neural Circuits. 2017. PMID: 28674487 Free PMC article.
-
Chronic 2P-STED imaging reveals high turnover of dendritic spines in the hippocampus in vivo.Elife. 2018 Jun 22;7:e34700. doi: 10.7554/eLife.34700. Elife. 2018. PMID: 29932052 Free PMC article.
-
Maternal Loss of Ube3a Impairs Experience-Driven Dendritic Spine Maintenance in the Developing Visual Cortex.J Neurosci. 2016 Apr 27;36(17):4888-94. doi: 10.1523/JNEUROSCI.4204-15.2016. J Neurosci. 2016. PMID: 27122043 Free PMC article.
-
KETAMINE: Neural- and network-level changes.Neuroscience. 2024 Nov 1;559:188-198. doi: 10.1016/j.neuroscience.2024.09.010. Epub 2024 Sep 7. Neuroscience. 2024. PMID: 39245312 Review.
Cited by
-
Cellular mechanisms and treatments for chemobrain: insight from aging and neurodegenerative diseases.EMBO Mol Med. 2020 Jun 8;12(6):e12075. doi: 10.15252/emmm.202012075. Epub 2020 Apr 29. EMBO Mol Med. 2020. PMID: 32346964 Free PMC article. Review.
-
Altered Cortical Ensembles in Mouse Models of Schizophrenia.Neuron. 2017 Apr 5;94(1):153-167.e8. doi: 10.1016/j.neuron.2017.03.019. Neuron. 2017. PMID: 28384469 Free PMC article.
-
Therapeutic mechanisms of psychedelics and entactogens.Neuropsychopharmacology. 2024 Jan;49(1):104-118. doi: 10.1038/s41386-023-01666-5. Epub 2023 Jul 24. Neuropsychopharmacology. 2024. PMID: 37488282 Free PMC article. Review.
-
Ketamine Rapidly Enhances Glutamate-Evoked Dendritic Spinogenesis in Medial Prefrontal Cortex Through Dopaminergic Mechanisms.Biol Psychiatry. 2021 Jun 1;89(11):1096-1105. doi: 10.1016/j.biopsych.2020.12.022. Epub 2021 Jan 8. Biol Psychiatry. 2021. PMID: 33637303 Free PMC article.
-
Anesthetics fragment hippocampal network activity, alter spine dynamics, and affect memory consolidation.PLoS Biol. 2021 Apr 1;19(4):e3001146. doi: 10.1371/journal.pbio.3001146. eCollection 2021 Apr. PLoS Biol. 2021. PMID: 33793545 Free PMC article.
References
-
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
