Altered Glycolysis and Mitochondrial Respiration in a Zebrafish Model of Dravet Syndrome
- PMID: 27066534
- PMCID: PMC4820792
- DOI: 10.1523/ENEURO.0008-16.2016
Altered Glycolysis and Mitochondrial Respiration in a Zebrafish Model of Dravet Syndrome
Abstract
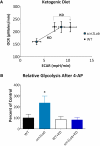
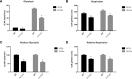
Altered metabolism is an important feature of many epileptic syndromes but has not been reported in Dravet syndrome (DS), a catastrophic childhood epilepsy associated with mutations in a voltage-activated sodium channel, Nav1.1 (SCN1A). To address this, we developed novel methodology to assess real-time changes in bioenergetics in zebrafish larvae between 4 and 6 d postfertilization (dpf). Baseline and 4-aminopyridine (4-AP) stimulated glycolytic flux and mitochondrial respiration were simultaneously assessed using a Seahorse Biosciences extracellular flux analyzer. Scn1Lab mutant zebrafish showed a decrease in baseline glycolytic rate and oxygen consumption rate (OCR) compared to controls. A ketogenic diet formulation rescued mutant zebrafish metabolism to control levels. Increasing neuronal excitability with 4-AP resulted in an immediate increase in glycolytic rates in wild-type zebrafish, whereas mitochondrial OCR increased slightly and quickly recovered to baseline values. In contrast, scn1Lab mutant zebrafish showed a significantly slower and exaggerated increase of both glycolytic rates and OCR after 4-AP. The underlying mechanism of decreased baseline OCR in scn1Lab mutants was not because of altered mitochondrial DNA content or dysfunction of enzymes in the electron transport chain or tricarboxylic acid cycle. Examination of glucose metabolism using a PCR array identified five glycolytic genes that were downregulated in scn1Lab mutant zebrafish. Our findings in scn1Lab mutant zebrafish suggest that glucose and mitochondrial hypometabolism contribute to the pathophysiology of DS.
Keywords: Dravet syndrome; epilepsy; glycolysis; metabolism; mitochondrial respiration; zebrafish.
Conflict of interest statement
The authors report no conflict of interest.
Figures




Similar articles
-
Behavioral Comorbidities and Drug Treatments in a Zebrafish scn1lab Model of Dravet Syndrome.eNeuro. 2017 Aug 14;4(4):ENEURO.0066-17.2017. doi: 10.1523/ENEURO.0066-17.2017. eCollection 2017 Jul-Aug. eNeuro. 2017. PMID: 28812061 Free PMC article.
-
New insights into the early mechanisms of epileptogenesis in a zebrafish model of Dravet syndrome.Epilepsia. 2020 Mar;61(3):549-560. doi: 10.1111/epi.16456. Epub 2020 Feb 24. Epilepsia. 2020. PMID: 32096222
-
A multielectrode array reveals therapeutic potential of translocator protein ligands in a zebrafish model of Dravet syndrome.J Pharmacol Exp Ther. 2025 Jul;392(7):103614. doi: 10.1016/j.jpet.2025.103614. Epub 2025 May 22. J Pharmacol Exp Ther. 2025. PMID: 40561753 Free PMC article.
-
Activity of drugs and components of natural origin in the severe myoclonic epilepsy of infancy (Dravet syndrome).Cent Nerv Syst Agents Med Chem. 2015;15(2):95-8. doi: 10.2174/1871524915666150430161321. Cent Nerv Syst Agents Med Chem. 2015. PMID: 25924876 Review.
-
Dravet syndrome: a genetic epileptic disorder.Acta Med Okayama. 2012;66(5):369-76. doi: 10.18926/AMO/48961. Acta Med Okayama. 2012. PMID: 23093055 Review.
Cited by
-
A bioenergetics assay for studying the effects of environmental stressors on mitochondrial function in vivo in zebrafish larvae.Comp Biochem Physiol C Toxicol Pharmacol. 2017 Feb;192:23-32. doi: 10.1016/j.cbpc.2016.12.001. Epub 2016 Dec 7. Comp Biochem Physiol C Toxicol Pharmacol. 2017. PMID: 27939721 Free PMC article.
-
The Generation of Human iPSC Lines from Three Individuals with Dravet Syndrome and Characterization of Neural Differentiation Markers in iPSC-Derived Ventral Forebrain Organoid Model.Cells. 2023 Jan 16;12(2):339. doi: 10.3390/cells12020339. Cells. 2023. PMID: 36672274 Free PMC article.
-
Metabolic Dysfunction and Oxidative Stress in Epilepsy.Int J Mol Sci. 2017 Nov 8;18(11):2365. doi: 10.3390/ijms18112365. Int J Mol Sci. 2017. PMID: 29117123 Free PMC article. Review.
-
Enhancing glucose metabolism via gluconeogenesis is therapeutic in a zebrafish model of Dravet syndrome.Brain Commun. 2021 Jan 25;3(1):fcab004. doi: 10.1093/braincomms/fcab004. eCollection 2021. Brain Commun. 2021. PMID: 33842883 Free PMC article.
-
Generation and Characterization of the Drosophila melanogaster paralytic Gene Knock-Out as a Model for Dravet Syndrome.Life (Basel). 2021 Nov 18;11(11):1261. doi: 10.3390/life11111261. Life (Basel). 2021. PMID: 34833136 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
