Epileptic encephalopathy-causing mutations in DNM1 impair synaptic vesicle endocytosis
- PMID: 27066543
- PMCID: PMC4821085
- DOI: 10.1212/01.NXG.0000464295.65736.da
Epileptic encephalopathy-causing mutations in DNM1 impair synaptic vesicle endocytosis
Abstract
Objective: To elucidate the functional consequences of epileptic encephalopathy-causing de novo mutations in DNM1 (A177P, K206N, G359A), which encodes a large mechanochemical GTPase essential for neuronal synaptic vesicle endocytosis.
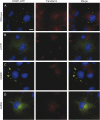
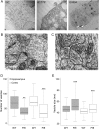
Methods: HeLa and COS-7 cells transfected with wild-type and mutant DNM1 constructs were used for transferrin assays, high-content imaging, colocalization studies, Western blotting, and electron microscopy (EM). EM was also conducted on the brain sections of mice harboring a middle-domain Dnm1 mutation (Dnm1 (Ftfl)).
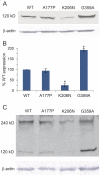
Results: We demonstrate that the expression of each mutant protein decreased endocytosis activity in a dominant-negative manner. One of the G-domain mutations, K206N, decreased protein levels. The G359A mutation, which occurs in the middle domain, disrupted higher-order DNM1 oligomerization. EM of mutant DNM1-transfected HeLa cells and of the Dnm1 (Ftfl) mouse brain revealed vesicle defects, indicating that the mutations likely interfere with DNM1's vesicle scission activity.
Conclusion: Together, these data suggest that the dysfunction of vesicle scission during synaptic vesicle endocytosis can lead to serious early-onset epilepsies.
Figures


References
-
- Dulac O. Epileptic encephalopathy. Epilepsia 2001;42(suppl 3):23–26. - PubMed
-
- Gray NW, Fourgeaud L, Huang B, et al. Dynamin 3 is a component of the postsynapse, where it interacts with mGluR5 and Homer. Curr Biol 2003;13:510–515. - PubMed
-
- Powell KA, Robinson PJ. Dephosphin/dynamin is a neuronal phosphoprotein concentrated in nerve terminals: evidence from rat cerebellum. Neuroscience 1995;64:821–833. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
