A Mouse Model of Zika Virus Pathogenesis
- PMID: 27066744
- PMCID: PMC4866885
- DOI: 10.1016/j.chom.2016.03.010
A Mouse Model of Zika Virus Pathogenesis
Abstract
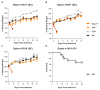
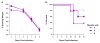
The ongoing Zika virus (ZIKV) epidemic and unexpected clinical outcomes, including Guillain-Barré syndrome and birth defects, has brought an urgent need for animal models. We evaluated infection and pathogenesis with contemporary and historical ZIKV strains in immunocompetent mice and mice lacking components of the antiviral response. Four- to six-week-old Irf3(-/-)Irf5(-/-)Irf7(-/-) triple knockout mice, which produce little interferon α/β, and mice lacking the interferon receptor (Ifnar1(-/-)) developed neurological disease and succumbed to ZIKV infection, whereas single Irf3(-/-), Irf5(-/-), and Mavs(-/-) knockout mice exhibited no overt illness. Ifnar1(-/-) mice sustained high viral loads in the brain and spinal cord, consistent with evidence that ZIKV causes neurodevelopmental defects in human fetuses. The testes of Ifnar1(-/-) mice had the highest viral loads, which is relevant to sexual transmission of ZIKV. This model of ZIKV pathogenesis will be valuable for evaluating vaccines and therapeutics as well as understanding disease pathogenesis.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


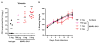

Comment in
-
Restriction of Zika Virus by Host Innate Immunity.Cell Host Microbe. 2016 May 11;19(5):566-7. doi: 10.1016/j.chom.2016.04.019. Cell Host Microbe. 2016. PMID: 27173920
-
Zika Virus Infection and Development of a Murine Model.Neurotox Res. 2016 Aug;30(2):131-4. doi: 10.1007/s12640-016-9635-3. Epub 2016 Jun 3. Neurotox Res. 2016. PMID: 27260223 Free PMC article.
References
-
- Alkan C, Zapata S, Bichaud L, Moureau G, Lemey P, Firth AE, Gritsun TS, Gould EA, de Lamballerie X, Depaquit J, et al. Ecuador Paraiso Escondido Virus, a New Flavivirus Isolated from New World Sand Flies in Ecuador, Is the First Representative of a Novel Clade in the Genus Flavivirus. J Virol. 2015;89:11773–11785. - PMC - PubMed
-
- Ank N, Iversen MB, Bartholdy C, Staeheli P, Hartmann R, Jensen UB, Dagnaes-Hansen F, Thomsen AR, Chen Z, Haugen H, et al. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J Immunol. 2008;180:2474–2485. - PubMed
-
- Bell TM, Field EJ, Narang HK. Zika virus infection of the central nervous system of mice. Archiv fur die gesamte Virusforschung. 1971;35:183–193. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

