Advances in cell surface glycoengineering reveal biological function
- PMID: 27066802
- PMCID: PMC5018048
- DOI: 10.1093/glycob/cww045
Advances in cell surface glycoengineering reveal biological function
Abstract
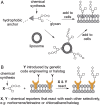
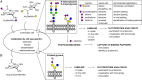
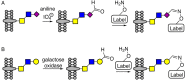
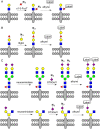
Cell surface glycans are critical mediators of cell-cell, cell-ligand, and cell-pathogen interactions. By controlling the set of glycans displayed on the surface of a cell, it is possible to gain insight into the biological functions of glycans. Moreover, control of glycan expression can be used to direct cellular behavior. While genetic approaches to manipulate glycosyltransferase gene expression are available, their utility in glycan engineering has limitations due to the combinatorial nature of glycan biosynthesis and the functional redundancy of glycosyltransferase genes. Biochemical and chemical strategies offer valuable complements to these genetic approaches, notably by enabling introduction of unnatural functionalities, such as fluorophores, into cell surface glycans. Here, we describe some of the most recent developments in glycoengineering of cell surfaces, with an emphasis on strategies that employ novel chemical reagents. We highlight key examples of how these advances in cell surface glycan engineering enable study of cell surface glycans and their function. Exciting new technologies include synthetic lipid-glycans, new chemical reporters for metabolic oligosaccharide engineering to allow tandem and in vivo labeling of glycans, improved chemical and enzymatic methods for glycoproteomics, and metabolic glycosyltransferase inhibitors. Many chemical and biochemical reagents for glycan engineering are commercially available, facilitating their adoption by the biological community.
Keywords: glycan labeling; glycoproteomics; glycosyltransferases; metabolic oligosaccharide engineering; metabolism.
© The Author 2016. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Chemical reporters to study mammalian O-glycosylation.Biochem Soc Trans. 2021 Apr 30;49(2):903-913. doi: 10.1042/BST20200839. Biochem Soc Trans. 2021. PMID: 33860782 Free PMC article. Review.
-
Expanding the Scope of Metabolic Glycan Labeling in Arabidopsis thaliana.Chembiochem. 2017 Jul 4;18(13):1286-1296. doi: 10.1002/cbic.201700069. Epub 2017 May 23. Chembiochem. 2017. PMID: 28383803
-
Glycosyltransferases as versatile tools to study the biology of glycans.Glycobiology. 2023 Dec 25;33(11):888-910. doi: 10.1093/glycob/cwad092. Glycobiology. 2023. PMID: 37956415 Review.
-
Metabolic Glyco-Engineering in Eukaryotic Cells and Selected Applications.Methods Mol Biol. 2015;1321:335-59. doi: 10.1007/978-1-4939-2760-9_23. Methods Mol Biol. 2015. PMID: 26082233
-
Tools for Studying Glycans: Recent Advances in Chemoenzymatic Glycan Labeling.ACS Chem Biol. 2017 Mar 17;12(3):611-621. doi: 10.1021/acschembio.6b01089. Epub 2017 Feb 13. ACS Chem Biol. 2017. PMID: 28301937 Free PMC article. Review.
Cited by
-
Time and space-resolved quantification of plasma membrane sialylation for measurements of cell function and neurotoxicity.Arch Toxicol. 2020 Feb;94(2):449-467. doi: 10.1007/s00204-019-02642-z. Epub 2019 Dec 11. Arch Toxicol. 2020. PMID: 31828357
-
Synthetic glycoscapes: addressing the structural and functional complexity of the glycocalyx.Interface Focus. 2019 Apr 6;9(2):20180080. doi: 10.1098/rsfs.2018.0080. Epub 2019 Feb 15. Interface Focus. 2019. PMID: 30842878 Free PMC article. Review.
-
Impacting Bacterial Sialidase Activity by Incorporating Bioorthogonal Chemical Reporters onto Mammalian Cell-Surface Sialosides.ACS Chem Biol. 2021 Nov 19;16(11):2307-2314. doi: 10.1021/acschembio.1c00469. Epub 2021 Sep 30. ACS Chem Biol. 2021. PMID: 34590826 Free PMC article.
-
Protected N-Acetyl Muramic Acid Probes Improve Bacterial Peptidoglycan Incorporation via Metabolic Labeling.ACS Chem Biol. 2021 Oct 15;16(10):1908-1916. doi: 10.1021/acschembio.1c00268. Epub 2021 Sep 10. ACS Chem Biol. 2021. PMID: 34506714 Free PMC article.
-
Labeling glycans on living cells by a chemoenzymatic glycoengineering approach.Biol Open. 2017 Jun 15;6(6):923-927. doi: 10.1242/bio.021600. Biol Open. 2017. PMID: 28500031 Free PMC article.
References
-
- Belcher JD, Chen C, Nguyen J, Abdulla F, Nguyen P, Nguyen M, Okeley NM, Benjamin DR, Senter PD, Vercellotti GM. 2015. The fucosylation inhibitor, 2-fluorofucose, inhibits vaso-occlusion, leukocyte-endothelium interactions and NF-kB activation in transgenic sickle mice. PLoS ONE. 10:e0117772. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

