The intersection of cell death and inflammasome activation
- PMID: 27066895
- PMCID: PMC11108284
- DOI: 10.1007/s00018-016-2205-2
The intersection of cell death and inflammasome activation
Abstract
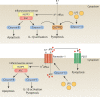
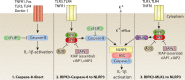
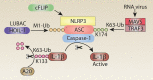
Inflammasomes sense cellular danger to activate the cysteine-aspartic protease caspase-1, which processes precursor interleukin-1β (IL-1β) and IL-18 into their mature bioactive fragments. In addition, activated caspase-1 or the related inflammatory caspase, caspase-11, can cleave gasdermin D to induce a lytic cell death, termed pyroptosis. The intertwining of IL-1β activation and cell death is further highlighted by research showing that the extrinsic apoptotic caspase, caspase-8, may, like caspase-1, directly process IL-1β, activate the NLRP3 inflammasome itself, or bind to inflammasome complexes to induce apoptotic cell death. Similarly, RIPK3- and MLKL-dependent necroptotic signaling can activate the NLRP3 inflammasome to drive IL-1β inflammatory responses in vivo. Here, we review the mechanisms by which cell death signaling activates inflammasomes to initiate IL-1β-driven inflammation, and highlight the clinical relevance of these findings to heritable autoinflammatory diseases. We also discuss whether the act of cell death can be separated from IL-1β secretion and evaluate studies suggesting that several cell death regulatory proteins can directly interact with, and modulate the function of, inflammasome and IL-1β containing protein complexes.
Keywords: Apoptosis; Caspase-1; Caspase-8; Inflammasome; MLKL; Necroptosis; Pyroptosis; RIPK3.
Figures
References
-
- Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10(2):417–426. pii: S1097276502005993 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

