Well-tolerated Spirulina extract inhibits influenza virus replication and reduces virus-induced mortality
- PMID: 27067133
- PMCID: PMC4828654
- DOI: 10.1038/srep24253
Well-tolerated Spirulina extract inhibits influenza virus replication and reduces virus-induced mortality
Abstract
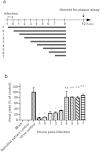
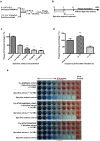
Influenza is one of the most common human respiratory diseases, and represents a serious public health concern. However, the high mutability of influenza viruses has hampered vaccine development, and resistant strains to existing anti-viral drugs have also emerged. Novel anti-influenza therapies are urgently needed, and in this study, we describe the anti-viral properties of a Spirulina (Arthrospira platensis) cold water extract. Anti-viral effects have previously been reported for extracts and specific substances derived from Spirulina, and here we show that this Spirulina cold water extract has low cellular toxicity, and is well-tolerated in animal models at one dose as high as 5,000 mg/kg, or 3,000 mg/kg/day for 14 successive days. Anti-flu efficacy studies revealed that the Spirulina extract inhibited viral plaque formation in a broad range of influenza viruses, including oseltamivir-resistant strains. Spirulina extract was found to act at an early stage of infection to reduce virus yields in cells and improve survival in influenza-infected mice, with inhibition of influenza hemagglutination identified as one of the mechanisms involved. Together, these results suggest that the cold water extract of Spirulina might serve as a safe and effective therapeutic agent to manage influenza outbreaks, and further clinical investigation may be warranted.
Figures



References
-
- Johnson N. P. & Mueller J. Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull. Hist. Med. 76, 105–115 (2002). - PubMed
-
- Dawood F. S. et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect. Dis. 12, 687–695 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

