Moxifloxacin's Limited Efficacy in the Hollow-Fiber Model of Mycobacterium abscessus Disease
- PMID: 27067317
- PMCID: PMC4879399
- DOI: 10.1128/AAC.02821-15
Moxifloxacin's Limited Efficacy in the Hollow-Fiber Model of Mycobacterium abscessus Disease
Abstract
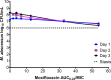
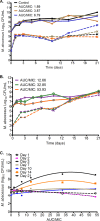
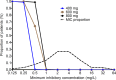
Current regimens used to treat pulmonary Mycobacterium abscessus disease have limited efficacy. There is an urgent need for new drugs and optimized combinations and doses. We performed hollow-fiber-system studies in which M. abscessus was exposed to moxifloxacin lung concentration-time profiles similar to human doses of between 0 and 800 mg/day. The minimum bactericidal concentration and MIC were 8 and 2 mg/liter, respectively, in our M. abscessus strain, suggesting bactericidal activity. Measurement of the moxifloxacin concentrations in each hollow-fiber system revealed an elimination rate constant (kel) of 0.11 ± 0.05 h(-1) (mean ± standard deviation) (half-life of 9.8 h). Inhibitory sigmoid maximal effect (Emax) modeling revealed that the highest Emax was 3.15 ± 1.84 log10 CFU/ml on day 3, and the exposure mediating 50% of Emax (EC50) was a 0- to 24-h area under the concentration time curve (AUC0-24)-to-MIC ratio of 41.99 ± 31.78 (r(2) = 0.99). The EC80 was an AUC0-24/MIC ratio of 102.11. However, no moxifloxacin concentration killed the bacteria to burdens below the starting inoculum. There was regrowth beyond day 3 in all doses, with replacement by a resistant subpopulation that had an MIC of >32 mg/liter by the end of the experiment. A quadratic function best described the relationship between the AUC0-24/MIC ratio and the moxifloxacin-resistant subpopulation. Monte Carlo simulations of 10,000 patients revealed that the 400- to 800-mg/day doses would achieve or exceed the EC80 in ≤12.5% of patients. The moxifloxacin susceptibility breakpoint was 0.25 mg/liter, which means that almost all M. abscessus clinical strains are moxifloxacin resistant by these criteria. While moxifloxacin's efficacy against M. abscessus was poor, formal combination therapy studies with moxifloxacin are still recommended.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi:10.1164/rccm.200604-571ST. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

