Clomipramine and Benznidazole Act Synergistically and Ameliorate the Outcome of Experimental Chagas Disease
- PMID: 27067322
- PMCID: PMC4879387
- DOI: 10.1128/AAC.00404-16
Clomipramine and Benznidazole Act Synergistically and Ameliorate the Outcome of Experimental Chagas Disease
Abstract
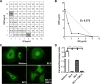
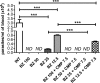
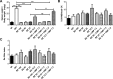
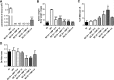
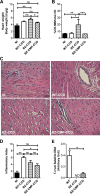
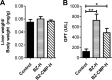
Chagas disease is an important public health problem in Latin America, and its treatment by chemotherapy with benznidazole (BZ) or nifurtimox remains unsatisfactory. In order to design new alternative strategies to improve the current etiological treatments, in the present work, we comprehensively evaluated the in vitro and in vivo anti-Trypanosoma cruzi effects of clomipramine (CMP) (a parasite-trypanothione reductase-specific inhibitor) combined with BZ. In vitro studies, carried out using a checkerboard technique on trypomastigotes (T. cruzi strain Tulahuen), revealed a combination index (CI) of 0.375, indicative of a synergistic effect of the drug combination. This result was correlated with the data obtained in infected BALB/c mice. We observed that during the acute phase (15 days postinfection [dpi]), BZ at 25 mg/kg of body weight/day alone decreased the levels of parasitemia compared with those of the control group, but when BZ was administered with CMP, the drug combination completely suppressed the parasitemia due to the observed synergistic effect. Furthermore, in the chronic phase (90 dpi), mice treated with both drugs showed less heart damage as assessed by the histopathological analysis, index of myocardial inflammation, and levels of heart injury biochemical markers than mice treated with BZ alone at the reference dose (100 mg/kg/day). Collectively, these data support the notion that CMP combined with low doses of BZ diminishes cardiac damage and inflammation during the chronic phase of cardiomyopathy. The synergistic activity of BZ-CMP clearly suggests a potential drug combination for Chagas disease treatment, which would allow a reduction of the effective dose of BZ and an increase in therapeutic safety.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
In vitro and in vivo drug combination for the treatment of Trypanosoma cruzi infection: A multivariate approach.Exp Parasitol. 2018 Jun;189:19-27. doi: 10.1016/j.exppara.2018.04.016. Epub 2018 Apr 18. Exp Parasitol. 2018. PMID: 29726395
-
In Vitro and In Vivo Trypanosomicidal Action of Novel Arylimidamides against Trypanosoma cruzi.Antimicrob Agents Chemother. 2016 Mar 25;60(4):2425-34. doi: 10.1128/AAC.01667-15. Print 2016 Apr. Antimicrob Agents Chemother. 2016. PMID: 26856830 Free PMC article.
-
Concomitant Benznidazole and Suramin Chemotherapy in Mice Infected with a Virulent Strain of Trypanosoma cruzi.Antimicrob Agents Chemother. 2015 Oct;59(10):5999-6006. doi: 10.1128/AAC.00779-15. Epub 2015 Jul 13. Antimicrob Agents Chemother. 2015. PMID: 26169419 Free PMC article.
-
Experimental models in Chagas disease: a review of the methodologies applied for screening compounds against Trypanosoma cruzi.Parasitol Res. 2018 Nov;117(11):3367-3380. doi: 10.1007/s00436-018-6084-3. Epub 2018 Sep 19. Parasitol Res. 2018. PMID: 30232605 Review.
-
[The chemotherapy of Chagas disease].Medicina (B Aires). 1999;59 Suppl 2:147-65. Medicina (B Aires). 1999. PMID: 10668258 Review. Spanish.
Cited by
-
Clinical significance of microbiota changes under the influence of psychotropic drugs. An updated narrative review.Front Microbiol. 2023 Mar 1;14:1125022. doi: 10.3389/fmicb.2023.1125022. eCollection 2023. Front Microbiol. 2023. PMID: 36937257 Free PMC article. Review.
-
Organic solvent-free benznidazole nanosuspension as an approach to a novel pediatric formulation for Chagas disease.Ther Deliv. 2024;15(9):699-716. doi: 10.1080/20415990.2024.2380244. Epub 2024 Aug 5. Ther Deliv. 2024. PMID: 39101355 Free PMC article.
-
Systematic screening of generic drugs for progressive multiple sclerosis identifies clomipramine as a promising therapeutic.Nat Commun. 2017 Dec 19;8(1):1990. doi: 10.1038/s41467-017-02119-6. Nat Commun. 2017. PMID: 29259169 Free PMC article.
-
Relevance of Trypanothione Reductase Inhibitors on Trypanosoma cruzi Infection: A Systematic Review, Meta-Analysis, and In Silico Integrated Approach.Oxid Med Cell Longev. 2018 Oct 24;2018:8676578. doi: 10.1155/2018/8676578. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30473742 Free PMC article.
-
Differential tissue distribution of discrete typing units after drug combination therapy in experimental Trypanosoma cruzi mixed infection.Parasitology. 2021 Nov;148(13):1595-1601. doi: 10.1017/S0031182021001281. Epub 2021 Jul 21. Parasitology. 2021. PMID: 35060468 Free PMC article.
References
-
- World Health Organization. 2015. Chagas disease. World Health Organization, Geneva, Switzerland: http://www.who.int/tdr/research/ntd/chagas/en/.
-
- Campi-Azevedo A, Gomes J, Teixeira-Carvalho A, Silveira-Lemos D, Vitelli-Avelar D, Sathler-Avelar R, Peruhype-Magalhães V, Béla S, Silvestre K, Batista M, Schachnik N, Correa-Oliveira R, Eloi-Santos S, Martins-Filho O. 2015. Etiological treatment of Chagas disease patients with benznidazole lead to a sustained pro-inflammatory profile counterbalanced by modulatory events. Immunobiology 220:564–574. doi: 10.1016/j.imbio.2014.12.006. - DOI - PubMed
-
- Moncayo A, Silveira A. 2009. Current epidemiological trends for Chagas disease in Latin America and future challenges in epidemiology, surveillance and health policy. Mem Inst Oswaldo Cruz 104(Suppl 1):17–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

