Aromatase expression and regulation in breast and endometrial cancer
- PMID: 27067638
- PMCID: PMC5519084
- DOI: 10.1530/JME-15-0310
Aromatase expression and regulation in breast and endometrial cancer
Abstract
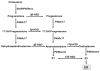
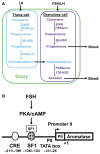
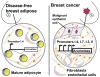
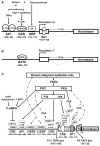
Long-term exposure to excess estrogen increases the risk of breast cancer and type 1 endometrial cancer. Most of the estrogen in premenopausal women is synthesized by the ovaries, while extraovarian subcutaneous adipose tissue is the predominant tissue source of estrogen after menopause. Estrogen and its metabolites can cause hyperproliferation and neoplastic transformation of breast and endometrial cells via increased proliferation and DNA damage. Several genetically modified mouse models have been generated to help understand the physiological and pathophysiological roles of aromatase and estrogen in the normal breast and in the development of breast cancers. Aromatase, the key enzyme for estrogen production, is comprised of at least ten partially tissue-selective and alternatively used promoters. These promoters are regulated by distinct signaling pathways to control aromatase expression and estrogen formation via recruitment of various transcription factors to their cis-regulatory elements. A shift in aromatase promoter use from I.4 to I.3/II is responsible for the excess estrogen production seen in fibroblasts surrounding malignant epithelial cells in breast cancers. Targeting these distinct pathways and/or transcription factors to modify aromatase activity may lead to the development of novel therapeutic remedies that inhibit estrogen production in a tissue-specific manner.
Keywords: aromatase; breast cancer; endometrial cancer; estrogen.
© 2016 Society for Endocrinology.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Figures

Similar articles
-
Regulation of aromatase expression in breast cancer tissue.Ann N Y Acad Sci. 2009 Feb;1155:121-31. doi: 10.1111/j.1749-6632.2009.03705.x. Ann N Y Acad Sci. 2009. PMID: 19250199 Review.
-
Aromatase excess in cancers of breast, endometrium and ovary.J Steroid Biochem Mol Biol. 2007 Aug-Sep;106(1-5):81-96. doi: 10.1016/j.jsbmb.2007.05.027. Epub 2007 May 24. J Steroid Biochem Mol Biol. 2007. PMID: 17590327 Free PMC article.
-
Use of alternative promoters to express the aromatase cytochrome P450 (CYP19) gene in breast adipose tissues of cancer-free and breast cancer patients.J Clin Endocrinol Metab. 1996 Nov;81(11):3843-9. doi: 10.1210/jcem.81.11.8923826. J Clin Endocrinol Metab. 1996. PMID: 8923826
-
BRCA1 negatively regulates the cancer-associated aromatase promoters I.3 and II in breast adipose fibroblasts and malignant epithelial cells.J Clin Endocrinol Metab. 2006 Nov;91(11):4514-9. doi: 10.1210/jc.2006-1364. Epub 2006 Aug 29. J Clin Endocrinol Metab. 2006. PMID: 16940443
-
The human CYP19 (aromatase P450) gene: update on physiologic roles and genomic organization of promoters.J Steroid Biochem Mol Biol. 2003 Sep;86(3-5):219-24. doi: 10.1016/s0960-0760(03)00359-5. J Steroid Biochem Mol Biol. 2003. PMID: 14623514 Review.
Cited by
-
Application of the Key Characteristics Framework to Identify Potential Breast Carcinogens Using Publicly Available in Vivo, in Vitro, and in Silico Data.Environ Health Perspect. 2024 Jan;132(1):17002. doi: 10.1289/EHP13233. Epub 2024 Jan 10. Environ Health Perspect. 2024. PMID: 38197648 Free PMC article.
-
Serial single-cell genomics reveals convergent subclonal evolution of resistance as early-stage breast cancer patients progress on endocrine plus CDK4/6 therapy.Nat Cancer. 2021 Jun;2(6):658-671. doi: 10.1038/s43018-021-00215-7. Epub 2021 Jun 3. Nat Cancer. 2021. PMID: 34712959 Free PMC article.
-
Structural Recognition and Binding Pattern Analysis of Human Topoisomerase II Alpha with Steroidal Drugs: In Silico Study to Switchover the Cancer Treatment.Asian Pac J Cancer Prev. 2020 May 1;21(5):1349-1355. doi: 10.31557/APJCP.2020.21.5.1349. Asian Pac J Cancer Prev. 2020. PMID: 32458643 Free PMC article.
-
Autophagy and senescence facilitate the development of antiestrogen resistance in ER positive breast cancer.Front Endocrinol (Lausanne). 2024 Mar 18;15:1298423. doi: 10.3389/fendo.2024.1298423. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38567308 Free PMC article. Review.
-
Regulation of aromatase in cancer.Mol Cell Biochem. 2021 Jun;476(6):2449-2464. doi: 10.1007/s11010-021-04099-0. Epub 2021 Feb 18. Mol Cell Biochem. 2021. PMID: 33599895 Review.
References
-
- Agarwal VR, Bulun SE, Leitch M, Rohrich R, Simpson ER. Use of alternative promoters to express the aromatase cytochrome P450 (CYP19) gene in breast adipose tissues of cancer-free and breast cancer patients. Journal of Clinical Endocrinology and Metabolism. 1996;81:3843–3849. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

