Galectin-3 in bone tumor microenvironment: a beacon for individual skeletal metastasis management
- PMID: 27067726
- PMCID: PMC6859002
- DOI: 10.1007/s10555-016-9622-4
Galectin-3 in bone tumor microenvironment: a beacon for individual skeletal metastasis management
Abstract
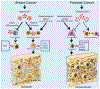
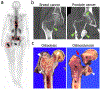
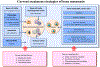
The skeleton is frequently a secondary growth site of disseminated cancers, often leading to painful and devastating clinical outcomes. Metastatic cancer distorts bone marrow homeostasis through tumor-derived factors, which shapes different bone tumor microenvironments depending on the tumor cells' origin. Here, we propose a novel insight on tumor-secreted Galectin-3 (Gal-3) that controls the induction of an inflammatory cascade, differentiation of osteoblasts, osteoclasts, and bone marrow cells, resulting in bone destruction and therapeutic failure. In the approaching era of personalized medicine, the current treatment modalities targeting bone metastatic environments are provided to the patient with limited consideration of the cancer cells' origin. Our new outlook suggests delivering individual tumor microenvironment treatments based on the expression level/activity/functionality of tumor-derived factors, rather than utilizing a commonly shared therapeutic umbrella. The notion of "Gal-3-associated bone remodeling" could be the first step toward a specific personalized therapy for each cancer type generating a different bone niche in patients afflicted with non-curable bone metastasis.
Keywords: Bone metastasis; Bone tumor microenvironment; Galectin-3; Personalized medicine.
Conflict of interest statement
Figures


Similar articles
-
Galectin-3 Cleavage Alters Bone Remodeling: Different Outcomes in Breast and Prostate Cancer Skeletal Metastasis.Cancer Res. 2016 Mar 15;76(6):1391-402. doi: 10.1158/0008-5472.CAN-15-1793. Epub 2016 Feb 2. Cancer Res. 2016. PMID: 26837763 Free PMC article.
-
Thrombospondins in bone remodeling and metastatic bone disease.Am J Physiol Cell Physiol. 2020 Dec 1;319(6):C980-C990. doi: 10.1152/ajpcell.00383.2020. Epub 2020 Sep 16. Am J Physiol Cell Physiol. 2020. PMID: 32936697 Review.
-
Targeting Intercellular Communication in the Bone Microenvironment to Prevent Disseminated Tumor Cell Escape from Dormancy and Bone Metastatic Tumor Growth.Int J Mol Sci. 2021 Mar 13;22(6):2911. doi: 10.3390/ijms22062911. Int J Mol Sci. 2021. PMID: 33805598 Free PMC article. Review.
-
Galectin-3 inhibits osteoblast differentiation through notch signaling.Neoplasia. 2014 Nov 20;16(11):939-49. doi: 10.1016/j.neo.2014.09.005. eCollection 2014 Nov. Neoplasia. 2014. PMID: 25425968 Free PMC article.
-
The role of galectin-3 in bone homeostasis: A review.Int J Biol Macromol. 2024 Oct;278(Pt 3):134882. doi: 10.1016/j.ijbiomac.2024.134882. Epub 2024 Aug 19. Int J Biol Macromol. 2024. PMID: 39168209 Review.
Cited by
-
Mechanistic Insight into the Antioxidant and Antimicrobial Activities of Palm Oil-Derived Biomaterials: Implications for Dental and Therapeutic Applications.Int J Mol Sci. 2025 Jul 20;26(14):6975. doi: 10.3390/ijms26146975. Int J Mol Sci. 2025. PMID: 40725222 Free PMC article. Review.
-
Survival and functional outcomes following surgical repair of pathological fractures in dogs: A meta-analysis.Open Vet J. 2025 May;15(5):2251-2258. doi: 10.5455/OVJ.2025.v15.i5.42. Epub 2025 May 31. Open Vet J. 2025. PMID: 40557067 Free PMC article.
-
Glycobiology in osteoclast differentiation and function.Bone Res. 2023 Oct 26;11(1):55. doi: 10.1038/s41413-023-00293-6. Bone Res. 2023. PMID: 37884496 Free PMC article. Review.
-
Amplification of autocrine motility factor and its receptor in multiple myeloma and other musculoskeletal tumors.J Bone Oncol. 2020 Jul 15;23:100308. doi: 10.1016/j.jbo.2020.100308. eCollection 2020 Aug. J Bone Oncol. 2020. PMID: 32714781 Free PMC article.
-
Association of Serum Galectin-3 with the Acute Exacerbation of Chronic Obstructive Pulmonary Disease.Med Sci Monit. 2017 Sep 26;23:4612-4618. doi: 10.12659/msm.903472. Med Sci Monit. 2017. PMID: 28947730 Free PMC article.
References
-
- Raz A, Bucana C, McLellan W, & Fidler IJ (1980). Distribution of membrane anionic sites on B16 melanoma variants with differing lung colonising potential. Nature, 284(5754), 363–364. - PubMed
-
- Raz A, & Lotan R (1981). Lectin-like activities associated with human and murine neoplastic cells. Cancer Research, 41(9 Pt 1), 3642–3647. - PubMed
-
- Liu FT, & Rabinovich GA (2005). Galectins as modulators of tumour progression. Nature Review. Cancer, 5(1), 29–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

