The Metalloprotease Mpl Supports Listeria monocytogenes Dissemination through Resolution of Membrane Protrusions into Vacuoles
- PMID: 27068088
- PMCID: PMC4907152
- DOI: 10.1128/IAI.00130-16
The Metalloprotease Mpl Supports Listeria monocytogenes Dissemination through Resolution of Membrane Protrusions into Vacuoles
Abstract
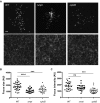
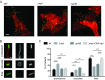
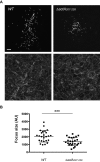
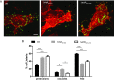
Listeria monocytogenes is an intracellular pathogen that disseminates within the intestinal epithelium through acquisition of actin-based motility and formation of plasma membrane protrusions that project into adjacent cells. The resolution of membrane protrusions into vacuoles from which the pathogen escapes results in bacterial spread from cell to cell. This dissemination process relies on the mlp-actA-plcB operon, which encodes ActA, a bacterial nucleation-promoting factor that mediates actin-based motility, and PlcB, a phospholipase that mediates vacuole escape. Here we investigated the role of the metalloprotease Mpl in the dissemination process. In agreement with previous findings showing that Mpl is required for PlcB activation, infection of epithelial cells with the ΔplcB or Δmpl strains resulted in the formation of small infection foci. As expected, the ΔplcB strain displayed a strong defect in vacuole escape. However, the Δmpl strain showed an unexpected defect in the resolution of protrusions into vacuoles, in addition to the expected but mild defect in vacuole escape. The Δmpl strain displayed increased levels of ActA on the bacterial surface in protrusions. We mapped an Mpl-dependent processing site in ActA between amino acid residues 207 to 238. Similar to the Δmpl strain, the ΔactA207-238 strain displayed increased levels of ActA on the bacterial surface in protrusions. Although the ΔactA207-238 strain displayed wild-type actin-based motility, it formed small infection foci and failed to resolve protrusions into vacuoles. We propose that, in addition to its role in PlcB processing and vacuole escape, the metalloprotease Mpl is required for ActA processing and protrusion resolution.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

