Dynamic interaction of SARAF with STIM1 and Orai1 to modulate store-operated calcium entry
- PMID: 27068144
- PMCID: PMC4828706
- DOI: 10.1038/srep24452
Dynamic interaction of SARAF with STIM1 and Orai1 to modulate store-operated calcium entry
Abstract
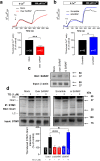
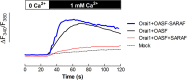
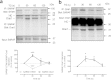
Ca(2+) influx by store-operated Ca(2+) channels is a major mechanism for intracellular Ca(2+) homeostasis and cellular function. Here we present evidence for the dynamic interaction between the SOCE-associated regulatory factor (SARAF), STIM1 and Orai1. SARAF overexpression attenuated SOCE and the STIM1-Orai1 interaction in cells endogenously expressing STIM1 and Orai1 while RNAi-mediated SARAF silencing induced opposite effects. SARAF impaired the association between Orai1 and the Orai1-activating small fragment of STIM1 co-expressed in the STIM1-deficient NG115-401L cells. Cell treatment with thapsigargin or physiological agonists results in direct association of SARAF with Orai1. STIM1-independent interaction of SARAF with Orai1 leads to activation of this channel. In cells endogenously expressing STIM1 and Orai1, Ca(2+) store depletion leads to dissociation of SARAF with STIM1 approximately 30s after treatment with thapsigargin, which paralleled the increase in SARAF-Orai1 interaction, followed by reinteraction with STIM1 and dissociation from Orai1. Co-expression of SARAF and either Orai1 or various N-terminal deletion Orai1 mutants did not alter SARAF-Orai1 interaction; however, expression of C-terminal deletion Orai1 mutants or blockade of the C-terminus of Orai1 impair the interaction with SARAF. These observations suggest that SARAF exerts an initial positive role in the activation of SOCE followed by the facilitation of SCDI of Orai1.
Figures

References
-
- Feske S. et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179–185 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

