Inherited and acquired disorders of myelin: The underlying myelin pathology
- PMID: 27068622
- PMCID: PMC5010953
- DOI: 10.1016/j.expneurol.2016.04.002
Inherited and acquired disorders of myelin: The underlying myelin pathology
Abstract
Remyelination is a major therapeutic goal in human myelin disorders, serving to restore function to demyelinated axons and providing neuroprotection. The target disorders that might be amenable to the promotion of this repair process are diverse and increasing in number. They range primarily from those of genetic, inflammatory to toxic origin. In order to apply remyelinating strategies to these disorders, it is essential to know whether the myelin damage results from a primary attack on myelin or the oligodendrocyte or both, and whether indeed these lead to myelin breakdown and demyelination. In some disorders, myelin sheath abnormalities are prominent but demyelination does not occur. This review explores the range of human and animal disorders where myelin pathology exists and focusses on defining the myelin changes in each and their cause, to help define whether they are targets for myelin repair therapy.
Keywords: Deficiency; Demyelination; Leukodystrophy; Multiple sclerosis; Myelin vacuolation; Toxin.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
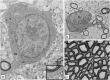




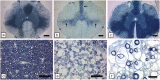







Comment in
-
Remyelination therapy for demyelinating disease.Nat Rev Neurol. 2020 Jun;16(6):346. doi: 10.1038/s41582-020-0341-7. Nat Rev Neurol. 2020. PMID: 32203392 No abstract available.
References
-
- Adachi M., Schneck L., Cara J., Volk B.W. Spongy degeneration of the central nervous system (van Bogaert and Bertrand type; Canavan's disease). A review. Hum. Pathol. 1973;4:331–347. - PubMed
-
- Agamanolis D.P., Victor M., Harris J.W., Hines J.D., Chester E.M., Kark J.A. An ultrastructural study of subacute combined degeneration of the spinal cord in vitamin B12-deficient rhesus monkeys. J. Neuropathol. Exp. Neurol. 1978;37:273–299. - PubMed
-
- Aubourg P. Axons need glial peroxisomes. Nat. Genet. 2007;39:936–938. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

