T-cell activation is an immune correlate of risk in BCG vaccinated infants
- PMID: 27068708
- PMCID: PMC4832066
- DOI: 10.1038/ncomms11290
T-cell activation is an immune correlate of risk in BCG vaccinated infants
Erratum in
-
Corrigendum: T-cell activation is an immune correlate of risk in BCG vaccinated infants.Nat Commun. 2016 May 6;7:11633. doi: 10.1038/ncomms11633. Nat Commun. 2016. PMID: 27151680 Free PMC article. No abstract available.
Abstract
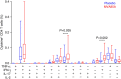
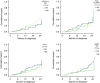
Vaccines to protect against tuberculosis (TB) are urgently needed. We performed a case-control analysis to identify immune correlates of TB disease risk in Bacille Calmette-Guerin (BCG) immunized infants from the MVA85A efficacy trial. Among 53 TB case infants and 205 matched controls, the frequency of activated HLA-DR(+) CD4(+) T cells associates with increased TB disease risk (OR=1.828, 95% CI=1.25-2.68, P=0.002, FDR=0.04, conditional logistic regression). In an independent study of Mycobacterium tuberculosis-infected adolescents, activated HLA-DR(+) CD4(+) T cells also associate with increased TB disease risk (OR=1.387, 95% CI=1.068-1.801, P=0.014, conditional logistic regression). In infants, BCG-specific T cells secreting IFN-γ associate with reduced risk of TB (OR=0.502, 95% CI=0.29-0.86, P=0.013, FDR=0.14). The causes and impact of T-cell activation on disease risk should be considered when designing and testing TB vaccine candidates for these populations.
Conflict of interest statement
H.M. was previously a shareholder in the Oxford-Emergent Tuberculosis Consortium (OETC), a joint venture established for the development of MVA85A (OETC no longer exists). The remaining authors declare no competing financial interests.
Figures



References
-
- Altare F. et al.. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280, 1432–1435 (1998). - PubMed
-
- Jouanguy E. et al.. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N. Engl. J. Med. 335, 1956–1961 (1996). - PubMed
-
- Lichtenauer-Kaligis E. G. et al.. Severe Mycobacterium bovis BCG infections in a large series of novel IL-12 receptor beta1 deficient patients and evidence for the existence of partial IL-12 receptor beta1 deficiency. Eur. J. Immunol. 33, 59–69 (2003). - PubMed
-
- Newport M. J. et al.. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 335, 1941–1949 (1996). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

