US Intergroup Trial of Response-Adapted Therapy for Stage III to IV Hodgkin Lymphoma Using Early Interim Fluorodeoxyglucose-Positron Emission Tomography Imaging: Southwest Oncology Group S0816
- PMID: 27069074
- PMCID: PMC4966513
- DOI: 10.1200/JCO.2015.63.1119
US Intergroup Trial of Response-Adapted Therapy for Stage III to IV Hodgkin Lymphoma Using Early Interim Fluorodeoxyglucose-Positron Emission Tomography Imaging: Southwest Oncology Group S0816
Abstract
Purpose: Four US National Clinical Trials Network components (Southwest Oncology Group, Cancer and Leukemia Group B/Alliance, Eastern Cooperative Oncology Group, and the AIDS Malignancy Consortium) conducted a phase II Intergroup clinical trial that used early interim fluorodeoxyglucose positron emission tomography (FDG-PET) imaging to determine the utility of response-adapted therapy for stage III to IV classic Hodgkin lymphoma.
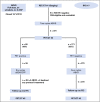
Patients and methods: The Southwest Oncology Group S0816 (Fludeoxyglucose F 18-PET/CT Imaging and Combination Chemotherapy With or Without Additional Chemotherapy and G-CSF in Treating Patients With Stage III or Stage IV Hodgkin Lymphoma) trial enrolled 358 HIV-negative patients between July 1, 2009, and December 2, 2012. A PET scan was performed after two initial cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) and was labeled PET2. PET2-negative patients (Deauville score 1 to 3) received an additional four cycles of ABVD, whereas PET2-positive patients (Deauville score 4 to 5) were switched to escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (eBEACOPP) for six cycles. Among 336 eligible and evaluable patients, the median age was 32 years (range, 18 to 60 years), with 52% stage III, 48% stage IV, 49% International Prognostic Score 0 to 2, and 51% score 3 to 7.
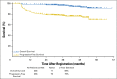
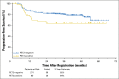
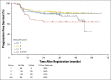
Results: Three hundred thirty-six of the enrolled patients were evaluable. Central review of the interim PET2 scan was performed in 331 evaluable patients, with 271 (82%) PET2-negative and 60 (18%) PET2-positive. Of 60 eligible PET2-positive patients, 49 switched to eBEACOPP as planned and 11 declined. With a median follow-up of 39.7 months, the Kaplan-Meier estimate for 2-year overall survival was 98% (95% CI, 95% to 99%), and the 2-year estimate for progression-free survival (PFS) was 79% (95% CI, 74% to 83%). The 2-year estimate for PFS in the subset of patients who were PET2-positive after two cycles of ABVD was 64% (95% CI, 50% to 75%). Both nonhematologic and hematologic toxicities were greater in the eBEACOPP arm than in the continued ABVD arm.
Conclusion: Response-adapted therapy based on interim PET imaging after two cycles of ABVD seems promising with a 2-year PFS of 64% for PET2-positive patients, which is much higher than the expected 2-year PFS of 15% to 30%.
© 2016 by American Society of Clinical Oncology.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at
Figures
Comment in
-
Balancing the Risk-Benefit Ratio in the Treatment of Patients With Advanced-Stage Hodgkin Lymphoma.J Clin Oncol. 2016 Jun 10;34(17):1975-7. doi: 10.1200/JCO.2015.66.3823. Epub 2016 Apr 11. J Clin Oncol. 2016. PMID: 27069072 No abstract available.
-
Reply to H.J.A. Adams et al and E.A. Hawkes et al.J Clin Oncol. 2017 Jan 20;35(3):373. doi: 10.1200/JCO.2016.69.7979. Epub 2016 Oct 28. J Clin Oncol. 2017. PMID: 28095266 No abstract available.
-
Is Upfront Escalated BEACOPP for Advanced Hodgkin Lymphoma Becoming a Distant Memory?J Clin Oncol. 2017 Jan 20;35(3):371-372. doi: 10.1200/JCO.2016.68.2047. Epub 2016 Oct 28. J Clin Oncol. 2017. PMID: 28095269 No abstract available.
-
Reply to H.J.A. Adams et al, E.A. Hawkes et al, and C.F. Hess et al.J Clin Oncol. 2017 Jan 20;35(3):375-376. doi: 10.1200/JCO.2016.69.7987. Epub 2016 Oct 28. J Clin Oncol. 2017. PMID: 28095272 No abstract available.
-
Predictive Value of Interim [18F]Fluorodeoxyglucose-Positron Emission Tomography in Advanced-Stage Hodgkin Lymphoma Is Not Well Established.J Clin Oncol. 2017 Jan 20;35(3):370-371. doi: 10.1200/JCO.2016.68.1494. Epub 2016 Oct 28. J Clin Oncol. 2017. PMID: 28095276 No abstract available.
-
Advanced Hodgkin's lymphoma: End-of-treatment FDG-PET should be maintained.Eur J Nucl Med Mol Imaging. 2017 Aug;44(8):1254-1257. doi: 10.1007/s00259-017-3714-4. Eur J Nucl Med Mol Imaging. 2017. PMID: 28466283 No abstract available.
References
-
- Press OW. Hodgkin lymphoma, in Kaushansky K, Lichtman M, Prchal J, et al (eds): Williams’ Hematology (ed 9). New York, NY, McGraw Hill, 2016, pp 1603-1624.
-
- Duggan DB, Petroni GR, Johnson JL, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin’s disease: Report of an intergroup trial. J Clin Oncol. 2003;21:607–614. - PubMed
-
- Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: An Intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol. 2013;31:684–691. - PMC - PubMed
-
- Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27:4548–4554. - PubMed
-
- Sieniawski M, Reineke T, Nogova L, et al. Fertility in male patients with advanced Hodgkin lymphoma treated with BEACOPP: A report of the German Hodgkin Study Group (GHSG). Blood. 2008;111:71–76. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- U10 CA180826/CA/NCI NIH HHS/United States
- UG1 CA189856/CA/NCI NIH HHS/United States
- UG1 CA189971/CA/NCI NIH HHS/United States
- U10 CA180834/CA/NCI NIH HHS/United States
- UG1 CA233329/CA/NCI NIH HHS/United States
- UG1 CA189821/CA/NCI NIH HHS/United States
- U10 CA180835/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U10 CA180801/CA/NCI NIH HHS/United States
- U10 CA180858/CA/NCI NIH HHS/United States
- UG1 CA189848/CA/NCI NIH HHS/United States
- UG1 CA189872/CA/NCI NIH HHS/United States
- RA/ARRA NIH HHS/United States
- U10 CA180846/CA/NCI NIH HHS/United States
- UG1 CA189858/CA/NCI NIH HHS/United States
- UG1 CA189860/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- UG1 CA189830/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- UG1 CA189854/CA/NCI NIH HHS/United States
- U10 CA180818/CA/NCI NIH HHS/United States
- U10 CA180828/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA180833/CA/NCI NIH HHS/United States
- UG1 CA189808/CA/NCI NIH HHS/United States
- U10 CA180799/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U10 CA180819/CA/NCI NIH HHS/United States
- U01 CA121947/CA/NCI NIH HHS/United States
- P30 CA006927/CA/NCI NIH HHS/United States
- U10 CA180816/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

