PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome
- PMID: 27069084
- PMCID: PMC5019753
- DOI: 10.1200/JCO.2016.66.4482
PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome
Abstract
Purpose: Classical Hodgkin lymphomas (cHLs) include small numbers of malignant Reed-Sternberg cells within an extensive but ineffective inflammatory/immune cell infiltrate. In cHL, chromosome 9p24.1/PD-L1/PD-L2 alterations increase the abundance of the PD-1 ligands, PD-L1 and PD-L2, and their further induction through Janus kinase 2-signal transducers and activators of transcription signaling. The unique composition of cHL limits its analysis with high-throughput genomic assays. Therefore, the precise incidence, nature, and prognostic significance of PD-L1/PD-L2 alterations in cHL remain undefined.
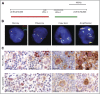
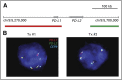
Methods: We used a fluorescent in situ hybridization assay to evaluate CD274/PD-L1 and PDCD1LG2/PD-L2 alterations in 108 biopsy specimens from patients with newly diagnosed cHL who were treated with the Stanford V regimen and had long-term follow-up. In each case, the frequency and magnitude of 9p24.1 alterations-polysomy, copy gain, and amplification-were determined, and the expression of PD-L1 and PD-L2 was evaluated by immunohistochemistry. We also assessed the association of 9p24.1 alterations with clinical parameters, which included stage (early stage I/II favorable risk, early stage unfavorable risk, advanced stage [AS] III/IV) and progression-free survival (PFS).
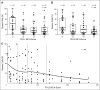
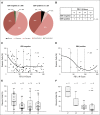
Results: Ninety-seven percent of all evaluated cHLs had concordant alterations of the PD-L1 and PD-L2 loci (polysomy, 5% [five of 108]; copy gain, 56% [61 of 108]; amplification, 36% [39 of 108]). There was an association between PD-L1 protein expression and relative genetic alterations in this series. PFS was significantly shorter for patients with 9p24.1 amplification, and the incidence of 9p24.1 amplification was increased in patients with AS cHL.
Conclusion: PD-L1/PD-L2 alterations are a defining feature of cHL. Amplification of 9p24.1 is more common in patients with AS disease and associated with shorter PFS in this series. Further analyses of 9p24.1 alterations in patients treated with standard cHL induction regimens or checkpoint blockade are warranted.
© 2016 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Figures



References
-
- Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: An intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol. 2013;31:684–691. - PMC - PubMed
-
- Hoskin PJ, Lowry L, Horwich A, et al. Randomized comparison of the Stanford V regimen and ABVD in the treatment of advanced Hodgkin’s Lymphoma: United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. J Clin Oncol. 2009;27:5390–5396. - PubMed
-
- Advani RH, Hong F, Fisher RI, et al. Randomized phase III trial comparing ABVD plus radiotherapy with the Stanford V regimen in patients with stages I or II locally extensive, bulky mediastinal Hodgkin lymphoma: A subset analysis of the North American Intergroup E2496 trial. J Clin Oncol. 2015;33:1936–1942. - PMC - PubMed
-
- Armitage JO. Early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363:653–662. - PubMed
-
- Kuruvilla J, Keating A, Crump M. How I treat relapsed and refractory Hodgkin lymphoma. Blood. 2011;117:4208–4217. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

