The BCL2 selective inhibitor venetoclax induces rapid onset apoptosis of CLL cells in patients via a TP53-independent mechanism
- PMID: 27069256
- PMCID: PMC4920022
- DOI: 10.1182/blood-2016-01-688796
The BCL2 selective inhibitor venetoclax induces rapid onset apoptosis of CLL cells in patients via a TP53-independent mechanism
Abstract
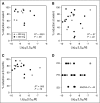
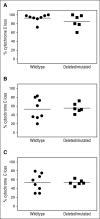
BCL2 blunts activation of the mitochondrial pathway to apoptosis, and high-level expression is required for chronic lymphocytic leukemia (CLL) survival. Venetoclax (ABT-199) is a small-molecule selective inhibitor of BCL2 currently in clinical trials for CLL and other malignancies. In conjunction with the phase 1 first-in-human clinical trial of venetoclax in patients with relapsed or refractory CLL (M12-175), we investigated the mechanism of action of venetoclax in vivo, explored whether in vitro sensitivity assays or BH3 profiling correlated with in vivo responses in patients, and determined whether loss of TP53 function affected responses in vitro and in vivo. In all samples tested, venetoclax induced death of CLL cells in vitro at concentrations achievable in vivo, with cell death evident within 4 hours. Apoptotic CLL cells were detected in vivo 6 or 24 hours after a single 20-mg or 50-mg dose in some patients. The extent of mitochondrial depolarization by a BIM BH3 peptide in vitro was correlated with percentage reduction of CLL in the blood and bone marrow in vivo, whereas the half lethal concentration derived from standard cytotoxicity assays was not. CLL cell death in vitro and the depth of clinical responses were independent of deletion of chromosome 17p, TP53 mutation, and TP53 function. These data provide direct evidence that venetoclax kills CLL cells in a TP53-independent fashion by inhibition of BCL2 in patients and support further assessment of BH3 profiling as a predictive biomarker for this drug.
© 2016 by The American Society of Hematology.
Figures





Comment in
-
To BH3 profile or not to BH3 profile.Blood. 2016 Jun 23;127(25):3111-2. doi: 10.1182/blood-2016-04-711762. Blood. 2016. PMID: 27340250 No abstract available.
References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. - PubMed
-
- Anderson MA, Huang D, Roberts A. Targeting BCL2 for the treatment of lymphoid malignancies. Semin Hematol. 2014;51(3):219–227. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

