Correlation of IL-18 with Tryptase in Atopic Asthma and Induction of Mast Cell Accumulation by IL-18
- PMID: 27069315
- PMCID: PMC4812453
- DOI: 10.1155/2016/4743176
Correlation of IL-18 with Tryptase in Atopic Asthma and Induction of Mast Cell Accumulation by IL-18
Abstract
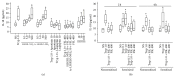
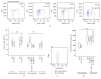
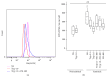
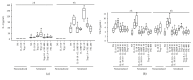
Interleukin- (IL-) 18 and tryptase were previously reported to relate to asthma, but the correlation between these two potent proinflammatory molecules in asthma and their roles in mast cell accumulation remain uninvestigated. Using flow cytometric analysis technique and ovalbumin- (OVA-) sensitized mouse model, it was found that IL-18 and tryptase levels in the plasma of moderate and severe asthma were elevated, and they correlated well with each other. Tryptase and agonist peptides of protease activated receptor- (PAR-) 2 induced substantial quantity of IL-18 release. IL-18 and tryptase provoked mast cell accumulation in peritoneum of OVA-sensitized mice. OVA-sensitization increased number of IL-18 receptor (R)(+) mast cells. IL-18 and tryptase induced dramatic increase in IL-18R(+) mast cells and mean fluorescence intensity (MFI) of IL-18R on mast cells. Moreover, while IL-18 induced an increase in PAR-2(+) mast cells in nonsensitized mice, IL-18 and tryptase provoked increases in IL-4 and thymic stromal lymphopoietin (TSLP) in the peritoneum of OVA-sensitized mice. In summary, the correlation between IL-18 and tryptase in plasma of patients with asthma indicates close interactions between them, which should be considered for development of anti-IL-18 and antitryptase therapies. Interactions between IL-18 and tryptase may contribute to mast cell recruitment in asthma.
Figures




References
-
- Ando M., Shima M. Serum interleukins 12 and 18 and immunoglobulin E concentrations and allergic symptoms in Japanese schoolchildren. Journal of Investigational Allergology and Clinical Immunology. 2007;17(1):14–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

