Cerium oxide nanoparticles promote neurogenesis and abrogate hypoxia-induced memory impairment through AMPK-PKC-CBP signaling cascade
- PMID: 27069362
- PMCID: PMC4818056
- DOI: 10.2147/IJN.S102096
Cerium oxide nanoparticles promote neurogenesis and abrogate hypoxia-induced memory impairment through AMPK-PKC-CBP signaling cascade
Retraction in
-
Cerium Oxide Nanoparticles Promote Neurogenesis and Abrogate Hypoxia-Induced Memory Impairment through AMPK-PKC-CBP Signaling Cascade [Retraction].Int J Nanomedicine. 2022 Nov 3;17:5163-5164. doi: 10.2147/IJN.S395863. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36353698 Free PMC article.
Abstract
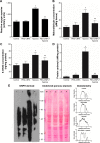
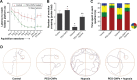
Structural and functional integrity of the brain is adversely affected by reduced oxygen saturation, especially during chronic hypoxia exposure and often encountered by altitude travelers or dwellers. Hypoxia-induced generation of reactive nitrogen and oxygen species reportedly affects the cortex and hippocampus regions of the brain, promoting memory impairment and cognitive dysfunction. Cerium oxide nanoparticles (CNPs), also known as nanoceria, switch between +3 and +4 oxidation states and reportedly scavenge superoxide anions, hydrogen peroxide, and peroxynitrite in vivo. In the present study, we evaluated the neuroprotective as well as the cognition-enhancing activities of nanoceria during hypobaric hypoxia. Using polyethylene glycol-coated 3 nm nanoceria (PEG-CNPs), we have demonstrated efficient localization of PEG-CNPs in rodent brain. This resulted in significant reduction of oxidative stress and associated damage during hypoxia exposure. Morris water maze-based memory function tests revealed that PEG-CNPs ameliorated hypoxia-induced memory impairment. Using microscopic, flow cytometric, and histological studies, we also provide evidences that PEG-CNPs augmented hippocampus neuronal survival and promoted neurogenesis. Molecular studies revealed that PEG-CNPs promoted neurogenesis through the 5'-adenine monophosphate-activated protein kinase-protein kinase C-cyclic adenosine monophosphate response element-binding protein binding (AMPK-PKC-CBP) protein pathway. Our present study results suggest that nanoceria can be translated as promising therapeutic molecules for neurodegenerative diseases.
Keywords: cerium oxide nanoparticles; hypoxia; memory; neuroprotection; oxidative stress.
Figures







References
-
- Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci. 2004;5(6):437–448. - PubMed
-
- Wilson MH, Newman S, Imray CH. The cerebral effects of ascent to high altitudes. Lancet Neurol. 2009;8(2):175–191. - PubMed
-
- LaManna JC, Chavez JC, Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J Exp Biol. 2004;207(Pt 18):3163–3169. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

