Neural Conversion and Patterning of Human Pluripotent Stem Cells: A Developmental Perspective
- PMID: 27069483
- PMCID: PMC4812494
- DOI: 10.1155/2016/8291260
Neural Conversion and Patterning of Human Pluripotent Stem Cells: A Developmental Perspective
Abstract
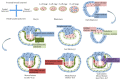
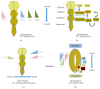
Since the reprogramming of adult human terminally differentiated somatic cells into induced pluripotent stem cells (hiPSCs) became a reality in 2007, only eight years have passed. Yet over this relatively short period, myriad experiments have revolutionized previous stem cell dogmata. The tremendous promise of hiPSC technology for regenerative medicine has fuelled rising expectations from both the public and scientific communities alike. In order to effectively harness hiPSCs to uncover fundamental mechanisms of disease, it is imperative to first understand the developmental neurobiology underpinning their lineage restriction choices in order to predictably manipulate cell fate to desired derivatives. Significant progress in developmental biology provides an invaluable resource for rationalising directed differentiation of hiPSCs to cellular derivatives of the nervous system. In this paper we begin by reviewing core developmental concepts underlying neural induction in order to provide context for how such insights have guided reductionist in vitro models of neural conversion from hiPSCs. We then discuss early factors relevant in neural patterning, again drawing upon crucial knowledge gained from developmental neurobiological studies. We conclude by discussing open questions relating to these concepts and how their resolution might serve to strengthen the promise of pluripotent stem cells in regenerative medicine.
Figures
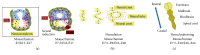


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

