Characterization of a multiprotein complex involved in excitation-transcription coupling of skeletal muscle
- PMID: 27069569
- PMCID: PMC4827232
- DOI: 10.1186/s13395-016-0087-5
Characterization of a multiprotein complex involved in excitation-transcription coupling of skeletal muscle
Abstract
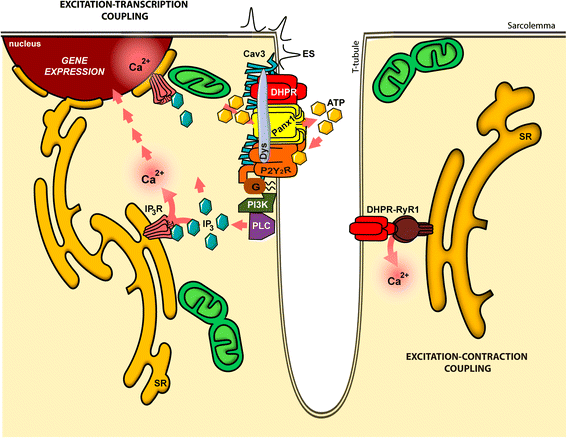
Background: Electrical activity regulates the expression of skeletal muscle genes by a process known as "excitation-transcription" (E-T) coupling. We have demonstrated that release of adenosine 5'-triphosphate (ATP) during depolarization activates membrane P2X/P2Y receptors, being the fundamental mediators between electrical stimulation, slow intracellular calcium transients, and gene expression. We propose that this signaling pathway would require the proper coordination between the voltage sensor (dihydropyridine receptor, DHPR), pannexin 1 channels (Panx1, ATP release conduit), nucleotide receptors, and other signaling molecules. The goal of this study was to assess protein-protein interactions within the E-T machinery and to look for novel constituents in order to characterize the signaling complex.
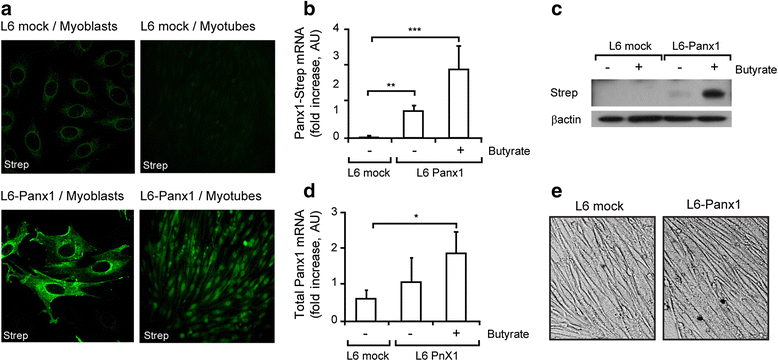
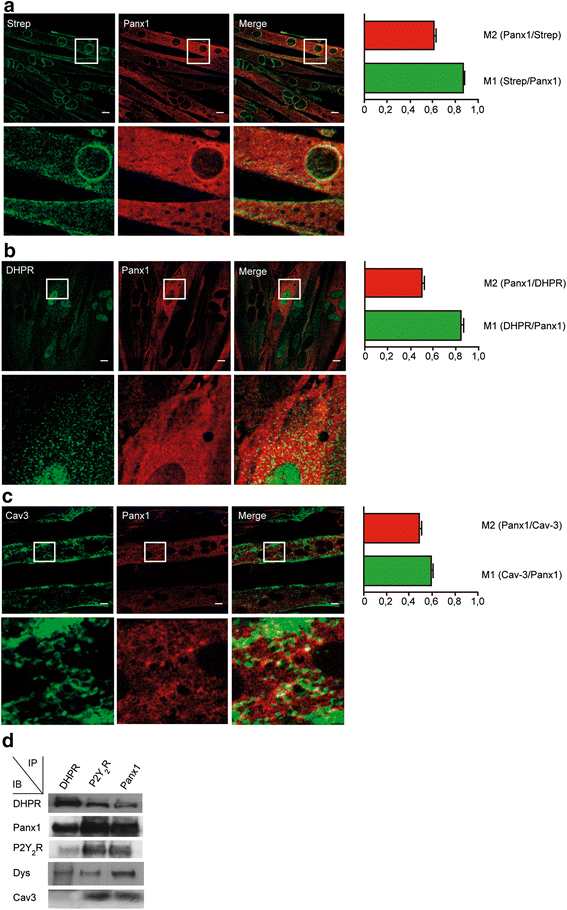
Methods: Newborn derived myotubes, adult fibers, or triad fractions from rat or mouse skeletal muscles were used. Co-immunoprecipitation, 2D blue native SDS/PAGE, confocal microscopy z-axis reconstruction, and proximity ligation assays were combined to assess the physical proximity of the putative complex interactors. An L6 cell line overexpressing Panx1 (L6-Panx1) was developed to study the influence of some of the complex interactors in modulation of gene expression.
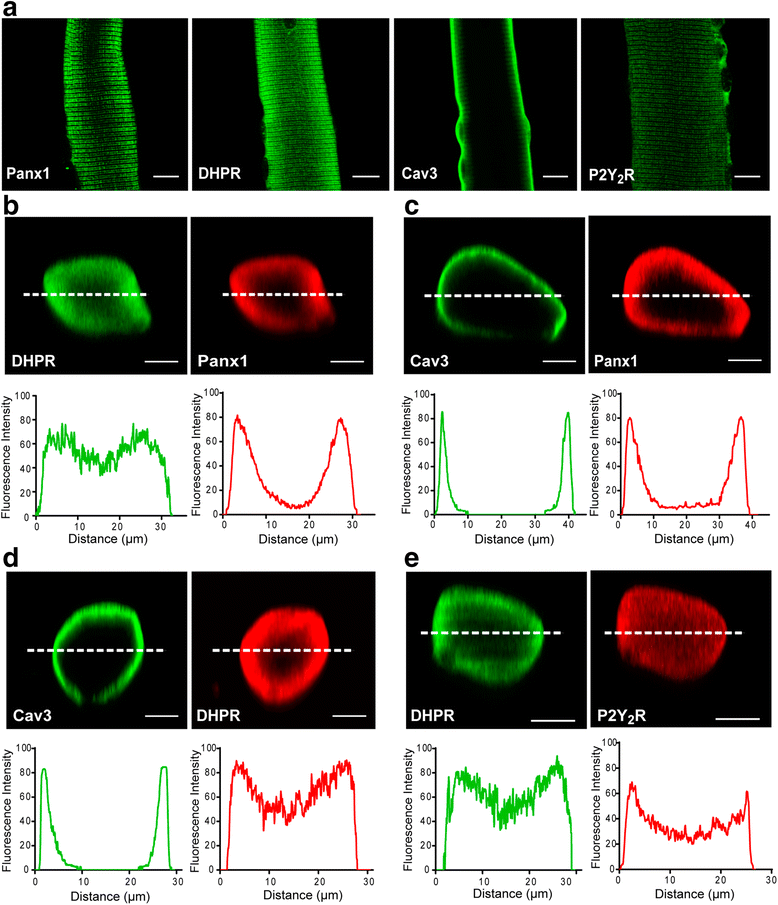
Results: Panx1, DHPR, P2Y2 receptor (P2Y2R), and dystrophin co-immunoprecipitated in the different preparations assessed. 2D blue native SDS/PAGE showed that DHPR, Panx1, P2Y2R and caveolin-3 (Cav3) belong to the same multiprotein complex. We observed co-localization and protein-protein proximity between DHPR, Panx1, P2Y2R, and Cav3 in adult fibers and in the L6-Panx1 cell line. We found a very restricted location of Panx1 and Cav3 in a putative T-tubule zone near the sarcolemma, while DHPR was highly expressed all along the transverse (T)-tubule. By Panx1 overexpression, extracellular ATP levels were increased both at rest and after electrical stimulation. Basal mRNA levels of the early gene cfos and the oxidative metabolism markers citrate synthase and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) were significantly increased by Panx1 overexpression. Interleukin 6 expression evoked by 20-Hz electrical stimulation (270 pulses, 0.3 ms each) was also significantly upregulated in L6-Panx1 cells.
Conclusions: We propose the existence of a relevant multiprotein complex that coordinates events involved in E-T coupling. Unveiling the molecular actors involved in the regulation of gene expression will contribute to the understanding and treatment of skeletal muscle disorders due to wrong-expressed proteins, as well as to improve skeletal muscle performance.
Keywords: Dihydropyridine receptor; Excitation-transcription coupling; Multiprotein complex; Nucleotide receptors; Pannexin 1; Skeletal muscle plasticity.
Figures





References
-
- Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab. 2000;279(4):E806–14. - PubMed
-
- Spina RJ, Chi MM, Hopkins MG, Nemeth PM, Lowry OH, Holloszy JO. Mitochondrial enzymes increase in muscle in response to 7–10 days of cycle exercise. J Appl Physiol. 1996;80(6):2250–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

