Genetic Deletion of ACE2 Induces Vascular Dysfunction in C57BL/6 Mice: Role of Nitric Oxide Imbalance and Oxidative Stress
- PMID: 27070147
- PMCID: PMC4829150
- DOI: 10.1371/journal.pone.0150255
Genetic Deletion of ACE2 Induces Vascular Dysfunction in C57BL/6 Mice: Role of Nitric Oxide Imbalance and Oxidative Stress
Abstract
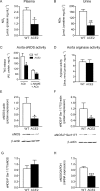
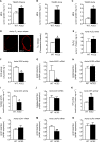
Accumulating evidence indicates that angiotensin-converting enzyme 2 (ACE2) plays a critical role in cardiovascular homeostasis, and its altered expression is associated with major cardiac and vascular disorders. The aim of this study was to evaluate the regulation of vascular function and assess the vascular redox balance in ACE2-deficient (ACE2-/y) animals. Experiments were performed in 20-22 week-old C57BL/6 and ACE2-/y male mice. Evaluation of endothelium-dependent and -independent relaxation revealed an impairment of in vitro and in vivo vascular function in ACE2-/y mice. Drastic reduction in eNOS expression at both protein and mRNA levels, and a decrease in •NO concentrations were observed in aortas of ACE2-/y mice in comparison to controls. Consistently, these mice presented a lower plasma and urine nitrite concentration, confirming reduced •NO availability in ACE2-deficient animals. Lipid peroxidation was significantly increased and superoxide dismutase activity was decreased in aorta homogenates of ACE2-/y mice, indicating impaired antioxidant capacity. Taken together, our data indicate, that ACE2 regulates vascular function by modulating nitric oxide release and oxidative stress. In conclusion, we elucidate mechanisms by which ACE2 is involved in the maintenance of vascular homeostasis. Furthermore, these findings provide insights into the role of the renin-angiotensin system in both vascular and systemic redox balance.
Conflict of interest statement
Figures


Similar articles
-
ACE2 and the Homolog Collectrin in the Modulation of Nitric Oxide and Oxidative Stress in Blood Pressure Homeostasis and Vascular Injury.Antioxid Redox Signal. 2017 Apr 20;26(12):645-659. doi: 10.1089/ars.2016.6950. Epub 2017 Jan 12. Antioxid Redox Signal. 2017. PMID: 27889958 Review.
-
Deletion of Rap1 disrupts redox balance and impairs endothelium-dependent relaxations.J Mol Cell Cardiol. 2018 Feb;115:1-9. doi: 10.1016/j.yjmcc.2017.12.009. Epub 2017 Dec 23. J Mol Cell Cardiol. 2018. PMID: 29277598
-
CD36/Sirtuin 1 Axis Impairment Contributes to Hepatic Steatosis in ACE2-Deficient Mice.Oxid Med Cell Longev. 2016;2016:6487509. doi: 10.1155/2016/6487509. Epub 2016 Dec 22. Oxid Med Cell Longev. 2016. PMID: 28101297 Free PMC article.
-
Angiotensin-converting enzyme 2 activation ameliorates pulmonary endothelial dysfunction in rats with pulmonary arterial hypertension through mediating phosphorylation of endothelial nitric oxide synthase.J Am Soc Hypertens. 2017 Dec;11(12):842-852. doi: 10.1016/j.jash.2017.10.009. Epub 2017 Oct 28. J Am Soc Hypertens. 2017. PMID: 29146157
-
ACE2-angiotensin-(1-7)-Mas axis and oxidative stress in cardiovascular disease.Hypertens Res. 2011 Feb;34(2):154-60. doi: 10.1038/hr.2010.235. Epub 2010 Dec 2. Hypertens Res. 2011. PMID: 21124322 Review.
Cited by
-
Elevated serum SDMA and ADMA at hospital admission predict in-hospital mortality of COVID-19 patients.Sci Rep. 2021 May 10;11(1):9895. doi: 10.1038/s41598-021-89180-w. Sci Rep. 2021. PMID: 33972591 Free PMC article.
-
Mechanisms of Cisplatin-Induced Acute Kidney Injury: Pathological Mechanisms, Pharmacological Interventions, and Genetic Mitigations.Cancers (Basel). 2021 Mar 29;13(7):1572. doi: 10.3390/cancers13071572. Cancers (Basel). 2021. PMID: 33805488 Free PMC article. Review.
-
ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome.J Microbiol Immunol Infect. 2020 Jun;53(3):425-435. doi: 10.1016/j.jmii.2020.04.015. Epub 2020 May 6. J Microbiol Immunol Infect. 2020. PMID: 32414646 Free PMC article. Review.
-
Genetics of macrovascular complications in type 2 diabetes.World J Diabetes. 2021 Aug 15;12(8):1200-1219. doi: 10.4239/wjd.v12.i8.1200. World J Diabetes. 2021. PMID: 34512887 Free PMC article. Review.
-
ACE2 Knockout Mice Are Resistant to High-Fat Diet-Induced Obesity in an Age-Dependent Manner.Int J Mol Sci. 2024 Sep 1;25(17):9515. doi: 10.3390/ijms25179515. Int J Mol Sci. 2024. PMID: 39273464 Free PMC article.
References
-
- Cosentino F, Barker JE, Brand MP, Heales SJ, Werner ER, Tippins JR, et al. (2001) Reactive oxygen species mediate endothelium-dependent relaxations in tetrahydrobiopterin-deficient mice. Arterioscler Thromb Vasc Biol 21: 496–502. - PubMed
-
- Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, et al. (2002) Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 417: 822–828. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

