In Silico Discovery of Potential Uridine-Cytidine Kinase 2 Inhibitors from the Rhizome of Alpinia mutica
- PMID: 27070566
- PMCID: PMC6274218
- DOI: 10.3390/molecules21040417
In Silico Discovery of Potential Uridine-Cytidine Kinase 2 Inhibitors from the Rhizome of Alpinia mutica
Abstract
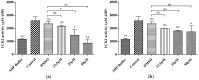
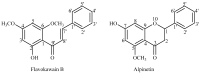
Uridine-cytidine kinase 2 is implicated in uncontrolled proliferation of abnormal cells and it is a hallmark of cancer, therefore, there is need for effective inhibitors of this key enzyme. In this study, we employed the used of in silico studies to find effective UCK2 inhibitors of natural origin using bioinformatics tools. An in vitro kinase assay was established by measuring the amount of ADP production in the presence of ATP and 5-fluorouridine as a substrate. Molecular docking studies revealed an interesting ligand interaction with the UCK2 protein for both flavokawain B and alpinetin. Both compounds were found to reduce ADP production, possibly by inhibiting UCK2 activity in vitro. In conclusion, we have identified flavokawain B and alpinetin as potential natural UCK2 inhibitors as determined by their interactions with UCK2 protein using in silico molecular docking studies. This can provide information to identify lead candidates for further drug design and development.
Keywords: Alpinia mutica; UCK2; alpinetin; amino acid active site residues; flavokawain B; in silico; nucleoside analogues.
Conflict of interest statement
All authors declared no conflict of interest.
Figures
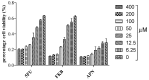




Similar articles
-
Crude Extracts, Flavokawain B and Alpinetin Compounds from the Rhizome of Alpinia mutica Induce Cell Death via UCK2 Enzyme Inhibition and in Turn Reduce 18S rRNA Biosynthesis in HT-29 Cells.PLoS One. 2017 Jan 19;12(1):e0170233. doi: 10.1371/journal.pone.0170233. eCollection 2017. PLoS One. 2017. PMID: 28103302 Free PMC article.
-
The pivotal role of uridine-cytidine kinases in pyrimidine metabolism and activation of cytotoxic nucleoside analogues in neuroblastoma.Biochim Biophys Acta. 2016 Sep;1862(9):1504-12. doi: 10.1016/j.bbadis.2016.05.012. Epub 2016 May 27. Biochim Biophys Acta. 2016. PMID: 27239701
-
Correction: Crude Extracts, Flavokawain B and Alpinetin Compounds from the Rhizome of Alpinia mutica Induce Cell Death via UCK2 Enzyme Inhibition and in Turn Reduce 18S rRNA Biosynthesis in HT-29 Cells.PLoS One. 2017 Mar 7;12(3):e0173651. doi: 10.1371/journal.pone.0173651. eCollection 2017. PLoS One. 2017. PMID: 28267789 Free PMC article.
-
Involvement of the uridine cytidine kinase 2 enzyme in cancer cell death: A molecular crosstalk between the enzyme and cellular apoptosis induction.Biomed Pharmacother. 2019 Jan;109:1506-1510. doi: 10.1016/j.biopha.2018.10.200. Epub 2018 Nov 14. Biomed Pharmacother. 2019. PMID: 30551402 Review.
-
Computational approaches for identifying potential inhibitors on targeting protein interactions in drug discovery.Adv Protein Chem Struct Biol. 2020;121:25-47. doi: 10.1016/bs.apcsb.2019.11.013. Epub 2020 Jan 13. Adv Protein Chem Struct Biol. 2020. PMID: 32312424 Review.
Cited by
-
Crude Extracts, Flavokawain B and Alpinetin Compounds from the Rhizome of Alpinia mutica Induce Cell Death via UCK2 Enzyme Inhibition and in Turn Reduce 18S rRNA Biosynthesis in HT-29 Cells.PLoS One. 2017 Jan 19;12(1):e0170233. doi: 10.1371/journal.pone.0170233. eCollection 2017. PLoS One. 2017. PMID: 28103302 Free PMC article.
-
Loss of disease tolerance during Citrobacter rodentium infection is associated with impaired epithelial differentiation and hyperactivation of T cell responses.Sci Rep. 2018 Jan 16;8(1):847. doi: 10.1038/s41598-017-17386-y. Sci Rep. 2018. PMID: 29339782 Free PMC article.
-
A microarray expression profile and bioinformatic analysis of circular RNA in human esophageal carcinoma.J Gastrointest Oncol. 2022 Apr;13(2):510-526. doi: 10.21037/jgo-22-137. J Gastrointest Oncol. 2022. PMID: 35557573 Free PMC article.
-
Natural Products Modulating Angiotensin Converting Enzyme 2 (ACE2) as Potential COVID-19 Therapies.Front Pharmacol. 2021 May 3;12:629935. doi: 10.3389/fphar.2021.629935. eCollection 2021. Front Pharmacol. 2021. PMID: 34012391 Free PMC article. Review.
-
Artonin E and Structural Analogs from Artocarpus Species Abrogates Estrogen Receptor Signaling in Breast Cancer.Molecules. 2016 Jun 29;21(7):839. doi: 10.3390/molecules21070839. Molecules. 2016. PMID: 27367662 Free PMC article.
References
-
- Wang F., Liu X., Liu C., Liu Z., Sun L. Effects of antibiotic antitumor drugs on nucleotide levels in cultured tumor cells: An exploratory method to distinguish the mechanisms of antitumor drug action based on targeted metabolomics. Acta Pharm. Sin. B. 2015;5:223–230. doi: 10.1016/j.apsb.2015.03.010. - DOI - PMC - PubMed
-
- Berg J.M., Tymoczko J.L., Stryer L. Biochemistry. 5th ed. W H Freeman; New York, NY, USA: 2002.
-
- Zhang C., Liu Z., Liu X., Wei L., Liu Y., Yu J., Sun L. Targeted metabolic analysis of nucleotides and identification of biomarkers associated with cancer in cultured cell models. Acta Pharm. Sin. B. 2013;3:254–262. doi: 10.1016/j.apsb.2013.06.002. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

