Absence of a Role for Phosphorylation in the Tau Pathology of Alzheimer's Disease
- PMID: 27070645
- PMCID: PMC4919914
- DOI: 10.3390/biom6020019
Absence of a Role for Phosphorylation in the Tau Pathology of Alzheimer's Disease
Erratum in
-
Erratum: Lai, R.Y.K.; Harrington, C.R.; Wischik, C.M. Absence of a Role for Phosphorylation in the Tau Pathology of Alzheimer's Disease. Biomolecules 2016, 6, 19.Biomolecules. 2016 Aug 12;6(3):35. doi: 10.3390/biom6030035. Biomolecules. 2016. PMID: 27529285 Free PMC article.
Abstract
Alzheimer's disease is characterized by redistribution of the tau protein pool from soluble to aggregated states. Aggregation forms proteolytically stable core polymers restricted to the repeat domain, and this binding interaction has prion-like properties. We have compared the binding properties of tau and tubulin in vitro using a system in which we can measure binding affinities for proteins alternated between solid and aqueous phases. The study reveals that a phase-shifted repeat domain fragment from the Paired Helical Filament core contains all that is required for high affinity tau-tau binding. Unlike tau-tubulin binding, tau-tau binding shows concentration-dependent enhancement in both phase directions due to an avidity effect which permits one molecule to bind to many as the concentration in the opposite phase increases. Phosphorylation of tau inhibits tau-tau binding and tau-tubulin binding to equivalent extents. Tau-tau binding is favoured over tau-tubulin binding by factors in the range 19-41-fold, irrespective of phosphorylation status. A critical requirement for tau to become aggregation-competent is prior binding to a solid-phase substrate, which induces a conformational change in the repeat domain permitting high-affinity binding to occur even if tau is phosphorylated. The endogenous species enabling this nucleation event to occur in vivo remains to be identified. The findings of the study suggest that development of disease-modifying drugs for tauopathies should not target phosphorylation, but rather should target inhibitors of tau-tau binding or inhibitors of the binding interaction with as yet unidentified endogenous polyanionic substrates required to nucleate tau assembly.
Keywords: Alzheimer’s disease; phosphorylation; protein aggregation; tau protein.
Figures
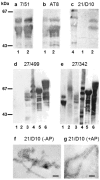

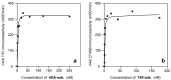



Similar articles
-
Oxidized and phosphorylated synthetic peptides corresponding to the second and third tubulin-binding repeats of the tau protein reveal structural features of paired helical filament assembly.J Pept Res. 1997 Aug;50(2):132-42. doi: 10.1111/j.1399-3011.1997.tb01178.x. J Pept Res. 1997. PMID: 9273897
-
Disease-Associated Tau Phosphorylation Hinders Tubulin Assembly within Tau Condensates.Angew Chem Int Ed Engl. 2021 Jan 11;60(2):726-730. doi: 10.1002/anie.202011157. Epub 2020 Nov 9. Angew Chem Int Ed Engl. 2021. PMID: 33017094 Free PMC article.
-
Tau mutants bind tubulin heterodimers with enhanced affinity.Proc Natl Acad Sci U S A. 2014 Apr 29;111(17):6311-6. doi: 10.1073/pnas.1315983111. Epub 2014 Apr 14. Proc Natl Acad Sci U S A. 2014. PMID: 24733915 Free PMC article.
-
Tau phosphorylation and aggregation in Alzheimer's disease pathology.FEBS Lett. 2006 May 22;580(12):2922-7. doi: 10.1016/j.febslet.2006.02.067. Epub 2006 Mar 3. FEBS Lett. 2006. PMID: 16529745 Review.
-
Ubiquitination and abnormal phosphorylation of paired helical filaments in Alzheimer's disease.Mol Neurobiol. 1991;5(2-4):399-410. doi: 10.1007/BF02935561. Mol Neurobiol. 1991. PMID: 1726645 Review.
Cited by
-
Tau Filament Self-Assembly and Structure: Tau as a Therapeutic Target.Front Neurol. 2020 Nov 12;11:590754. doi: 10.3389/fneur.2020.590754. eCollection 2020. Front Neurol. 2020. PMID: 33281730 Free PMC article. Review.
-
Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer's disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial.Lancet. 2016 Dec 10;388(10062):2873-2884. doi: 10.1016/S0140-6736(16)31275-2. Epub 2016 Nov 16. Lancet. 2016. PMID: 27863809 Free PMC article. Clinical Trial.
-
Imbalanced Expression of Tau and Tubulin Induces Neuronal Dysfunction in C. elegans Models of Tauopathy.Front Neurosci. 2018 Jun 20;12:415. doi: 10.3389/fnins.2018.00415. eCollection 2018. Front Neurosci. 2018. PMID: 29973863 Free PMC article.
-
Differential compartmental processing and phosphorylation of pathogenic human tau and native mouse tau in the line 66 model of frontotemporal dementia.J Biol Chem. 2020 Dec 25;295(52):18508-18523. doi: 10.1074/jbc.RA120.014890. Epub 2020 Oct 30. J Biol Chem. 2020. PMID: 33127647 Free PMC article.
-
Role of Tau Acetylation in Alzheimer's Disease and Chronic Traumatic Encephalopathy: The Way Forward for Successful Treatment.J Neurol Neurosurg. 2017;4(2):140. Epub 2017 Dec 7. J Neurol Neurosurg. 2017. PMID: 29276758 Free PMC article.
References
-
- Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. Allg. Z. Psych. Psych. Gerich. Med. 1907;64:146–148.
-
- Nelson P.T., Alafuzoff I., Bigio E.H., Bouras C., Braak H., Cairns N.J., Castellani R.J., Crain B.J., Davies P., Tredici K.D., et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J. Neuropathol. Exp. Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

