Screening Coverage Needed to Reduce Mortality from Prostate Cancer: A Living Systematic Review
- PMID: 27070904
- PMCID: PMC4829241
- DOI: 10.1371/journal.pone.0153417
Screening Coverage Needed to Reduce Mortality from Prostate Cancer: A Living Systematic Review
Abstract
Introduction: Screening for prostate cancer remains controversial because of conflicting results from the two major trials: The Prostate, Lung, Colorectal and Ovarian Cancer (PLCO) screening trial and the European Randomized Study of Screening for Prostate Cancer (ERSPC).
Objective: Meta-analyze and meta-regress the available PSA screening trials.
Methods: We performed a living systematic review and meta-regression of the reduction in prostate cancer mortality as a function of the duration of screening provided in each trial. We searched PubMed, Web of Science, the Cochrane Registry, and references lists from previous meta-analyses to identify randomized trials of PSA screening. We followed PRISMA guidelines and qualified strength of evidence with a GRADE Profile.
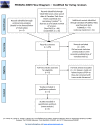
Results: We found 6 trials, but excluded one that also screened with trans-rectal ultrasound. We considered each ERSPC center as a separate trial. When pooling together all 11 trials we found no significant benefit from screening; however, the heterogeneity was 28.2% (95% CI: 0% to 65%). Heterogeneity was explained by variations in the duration of serial screening (I2 0%; 95% CI: 0% to 52%). When we analyzed the subgroup of trials that added more than 3 years of screening (range 3.2 to 3.8) we found a significant benefit for screening with risk ratio 0.78 (95% CI 0.65-0.94; I2 = 0%; 95% CI: 0% to 69%) and a number needed to invite for screening of 1000. We downgraded the quality of evidence to moderate due to our retrospective identification of subgroups and limited data on control group screening.
Conclusions: Adequate duration of screening reduces mortality from prostate cancer. The benefit, while small, compares favorably with screening for other cancers. Our projections are limited by the moderate quality of evidence.
Conflict of interest statement
Figures




Similar articles
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Interventions for promoting habitual exercise in people living with and beyond cancer.Cochrane Database Syst Rev. 2018 Sep 19;9(9):CD010192. doi: 10.1002/14651858.CD010192.pub3. Cochrane Database Syst Rev. 2018. PMID: 30229557 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Bisphosphonates or RANK-ligand-inhibitors for men with prostate cancer and bone metastases: a network meta-analysis.Cochrane Database Syst Rev. 2020 Dec 3;12(12):CD013020. doi: 10.1002/14651858.CD013020.pub2. Cochrane Database Syst Rev. 2020. PMID: 33270906 Free PMC article.
Cited by
-
Sensitive detection of HSP70 using a current-amplified biosensor based on antibody-loaded PS-AuNPs@Cys/Au modified ITO chip.Mikrochim Acta. 2024 Apr 18;191(5):272. doi: 10.1007/s00604-024-06333-0. Mikrochim Acta. 2024. PMID: 38634999
-
The knowledge and attitude towards prostate cancer and screening practices among males in Saudi Arabia.J Family Med Prim Care. 2022 Jun;11(6):2637-2642. doi: 10.4103/jfmpc.jfmpc_1802_21. Epub 2022 Jun 30. J Family Med Prim Care. 2022. PMID: 36119286 Free PMC article.
-
Knowledge, attitudes, and practices towards prostate cancer screening amongst men living in the southern Italian peninsula: the Prevention and Research in Oncology (PRO) non-profit Foundation experience.World J Urol. 2017 Dec;35(12):1857-1862. doi: 10.1007/s00345-017-2074-9. Epub 2017 Aug 5. World J Urol. 2017. PMID: 28780740
-
A living critical interpretive synthesis to yield a framework on the production and dissemination of living evidence syntheses for decision-making.Implement Sci. 2024 Sep 27;19(1):67. doi: 10.1186/s13012-024-01396-2. Implement Sci. 2024. PMID: 39334425 Free PMC article. Review.
-
Clinical practice guidelines for molecular tumor marker, 2nd edition review part 2.Int J Clin Oncol. 2024 May;29(5):512-534. doi: 10.1007/s10147-024-02497-0. Epub 2024 Mar 17. Int J Clin Oncol. 2024. PMID: 38493447
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

