The Association between Sulfonylurea Use and All-Cause and Cardiovascular Mortality: A Meta-Analysis with Trial Sequential Analysis of Randomized Clinical Trials
- PMID: 27071029
- PMCID: PMC4829174
- DOI: 10.1371/journal.pmed.1001992
The Association between Sulfonylurea Use and All-Cause and Cardiovascular Mortality: A Meta-Analysis with Trial Sequential Analysis of Randomized Clinical Trials
Erratum in
-
Correction: The Association between Sulfonylurea Use and All-Cause and Cardiovascular Mortality: A Meta-Analysis with Trial Sequential Analysis of Randomized Clinical Trials.PLoS Med. 2016 Jun 24;13(6):e1002091. doi: 10.1371/journal.pmed.1002091. eCollection 2016 Jun. PLoS Med. 2016. PMID: 27340828 Free PMC article.
Abstract
Background: Sulfonylureas are an effective and inexpensive treatment for type 2 diabetes. There is conflicting data about the safety of these drugs regarding mortality and cardiovascular outcomes. The objective of the present study was to evaluate the safety of the sulfonylureas most frequently used and to use trial sequential analysis (TSA) to analyze whether the available sample was powered enough to support the results.
Methods and findings: Electronic databases were reviewed from 1946 (Embase) or 1966 (MEDLINE) up to 31 December 2014. Randomized clinical trials (RCTs) of at least 52 wk in duration evaluating second- or third-generation sulfonylureas in the treatment of adults with type 2 diabetes and reporting outcomes of interest were included. Primary outcomes were all-cause and cardiovascular mortality. Additionally, myocardial infarction and stroke events were evaluated. Data were summarized with Peto odds ratios (ORs), and the reliability of the results was evaluated with TSA. Forty-seven RCTs with 37,650 patients and 890 deaths in total were included. Sulfonylureas were not associated with all-cause (OR 1.12 [95% CI 0.96 to 1.30]) or cardiovascular mortality (OR 1.12 [95% CI 0.87 to 1.42]). Sulfonylureas were also not associated with increased risk of myocardial infarction (OR 0.92 [95% CI 0.76 to 1.12]) or stroke (OR 1.16 [95% CI 0.81 to 1.66]). TSA could discard an absolute difference of 0.5% between the treatments, which was considered the minimal clinically significant difference. The major limitation of this review was the inclusion of studies not designed to evaluate safety outcomes.
Conclusions: Sulfonylureas are not associated with increased risk for all-cause mortality, cardiovascular mortality, myocardial infarction, or stroke. Current evidence supports the safety of sulfonylureas; an absolute risk of 0.5% could be firmly discarded.
Review registration: PROSPERO CRD42014004330.
Conflict of interest statement
JLG reports grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico, during the conduct of the study; grants and other from Eli Lilly, grants from Bristol-Myers Squibb, grants and other from Boehringer Ingelheim, grants from GlaxoSmithKline, grants and other from Novo Nordisk, grants from Janssen, outside the submitted work; no other relationships or activities that could appear to have influenced the submitted work are reported. DVR, LCP, LRR and CBL have declared that no competing interests exist.
Figures
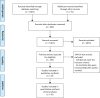





References
-
- (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352: 837–853. - PubMed
-
- Meinert CL, Knatterud GL, Prout TE, Klimt CR (1970) A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results. Diabetes 19 (Suppl): 789–830. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

