Phase 1 Study of a Sulforaphane-Containing Broccoli Sprout Homogenate for Sickle Cell Disease
- PMID: 27071063
- PMCID: PMC4829228
- DOI: 10.1371/journal.pone.0152895
Phase 1 Study of a Sulforaphane-Containing Broccoli Sprout Homogenate for Sickle Cell Disease
Abstract
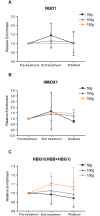
Sickle cell disease (SCD) is the most common inherited hemoglobinopathy worldwide. Our previous results indicate that the reduced oxidative stress capacity of sickle erythrocytes may be caused by decreased expression of NRF2 (Nuclear factor (erythroid-derived 2)-like 2), an oxidative stress regulator. We found that activation of NRF2 with sulforaphane (SFN) in erythroid progenitors significantly increased the expression of NRF2 targets HMOX1, NQO1, and HBG1 (subunit of fetal hemoglobin) in a dose-dependent manner. Therefore, we hypothesized that NRF2 activation with SFN may offer therapeutic benefits for SCD patients by restoring oxidative capacity and increasing fetal hemoglobin concentration. To test this hypothesis, we performed a Phase 1, open-label, dose-escalation study of SFN, contained in a broccoli sprout homogenate (BSH) that naturally contains SFN, in adults with SCD. The primary and secondary study endpoints were safety and physiological response to NRF2 activation, respectively. We found that BSH was well tolerated, and the few adverse events that occurred during the trial were not likely related to BSH consumption. We observed an increase in the mean relative whole blood mRNA levels for the NRF2 target HMOX1 (p = 0.02) on the last day of BSH treatment, compared to pre-treatment. We also observed a trend toward increased mean relative mRNA levels of the NRF2 target HBG1 (p = 0.10) from baseline to end of treatment, but without significant changes in HbF protein. We conclude that BSH, in the provided doses, is safe in stable SCD patients and may induce changes in gene expression levels. We therefore propose investigation of more potent NRF2 inducers, which may elicit more robust physiological changes and offer clinical benefits to SCD patients. Trial registration: ClinicalTrials.gov NCT01715480.
Conflict of interest statement
Figures


References
-
- Prevention CfDCa. Sickle Cell Disease: Data & Statistics 16 September 2011 Accessed: 18 April 2015.
-
- Pauling L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a molecular disease. Science. 1949;109(2835):443 . - PubMed
-
- Kaul DK, Tsai HM, Liu XD, Nakada MT, Nagel RL, Coller BS. Monoclonal antibodies to alphaVbeta3 (7E3 and LM609) inhibit sickle red blood cell-endothelium interactions induced by platelet-activating factor. Blood. 2000;95(2):368–74. . - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

