Soluble guanylate cyclase as an alternative target for bronchodilator therapy in asthma
- PMID: 27071111
- PMCID: PMC4855555
- DOI: 10.1073/pnas.1524398113
Soluble guanylate cyclase as an alternative target for bronchodilator therapy in asthma
Abstract
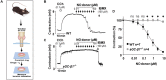
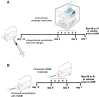
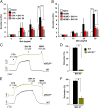
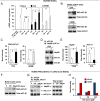
Asthma is defined by airway inflammation and hyperresponsiveness, and contributes to morbidity and mortality worldwide. Although bronchodilation is a cornerstone of treatment, current bronchodilators become ineffective with worsening asthma severity. We investigated an alternative pathway that involves activating the airway smooth muscle enzyme, soluble guanylate cyclase (sGC). Activating sGC by its natural stimulant nitric oxide (NO), or by pharmacologic sGC agonists BAY 41-2272 and BAY 60-2770, triggered bronchodilation in normal human lung slices and in mouse airways. Both BAY 41-2272 and BAY 60-2770 reversed airway hyperresponsiveness in mice with allergic asthma and restored normal lung function. The sGC from mouse asthmatic lungs displayed three hallmarks of oxidative damage that render it NO-insensitive, and identical changes to sGC occurred in human lung slices or in human airway smooth muscle cells when given chronic NO exposure to mimic the high NO in asthmatic lung. Our findings show how allergic inflammation in asthma may impede NO-based bronchodilation, and reveal that pharmacologic sGC agonists can achieve bronchodilation despite this loss.
Keywords: S-nitrosylation; bronchoconstriction; bronchodilation; heme protein; nitric oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Olin JT, Wechsler ME. Asthma: Pathogenesis and novel drugs for treatment. BMJ. 2014;349:g5517. - PubMed
-
- Ellis JL. Role of soluble guanylyl cyclase in the relaxations to a nitric oxide donor and to nonadrenergic nerve stimulation in guinea pig trachea and human bronchus. J Pharmacol Exp Ther. 1997;280(3):1215–1218. - PubMed
-
- Papapetropoulos A, Simoes DC, Xanthou G, Roussos C, Gratziou C. Soluble guanylyl cyclase expression is reduced in allergic asthma. Am J Physiol Lung Cell Mol Physiol. 2006;290(1):L179–L184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

