SAXS studies of RNA: structures, dynamics, and interactions with partners
- PMID: 27071649
- PMCID: PMC4909577
- DOI: 10.1002/wrna.1349
SAXS studies of RNA: structures, dynamics, and interactions with partners
Abstract
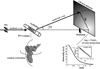
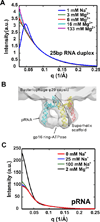
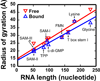
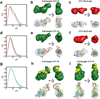
Small-angle X-ray scattering, SAXS, is a powerful and easily employed experimental technique that provides solution structures of macromolecules. The size and shape parameters derived from SAXS provide global structural information about these molecules in solution and essentially complement data acquired by other biophysical methods. As applied to protein systems, SAXS is a relatively mature technology: sophisticated tools exist to acquire and analyze data, and to create structural models that include dynamically flexible ensembles. Given the expanding appreciation of RNA's biological roles, there is a need to develop comparable tools to characterize solution structures of RNA, including its interactions with important biological partners. We review the progress toward achieving this goal, focusing on experimental and computational innovations. The use of multiphase modeling, absolute calibration and contrast variation methods, among others, provides new and often unique ways of visualizing this important biological molecule and its essential partners: ions, other RNAs, or proteins. WIREs RNA 2016, 7:512-526. doi: 10.1002/wrna.1349 For further resources related to this article, please visit the WIREs website.
© 2016 Wiley Periodicals, Inc.
Figures


References
-
- Svergun DI, Koch MHJ. Small-angle scattering studies of biological macromolecules in solution. Reports on Progress in Physics. 2003;66:1735–1782.
-
- Lipfert J, Doniach S. Small-angle X-ray scattering from RNA, proteins, and protein complexes. Annual Review of Biophysics and Biomolecular Structure. 2007;36:307–327. - PubMed
-
- Doniach S, Lipfert J. Use of Small Angle X-Ray Scattering (Saxs) to Characterize Conformational States of Functional RNAs. Methods in Enzymology, Vol 469: Biophysical, Chemical, and Functional Probes of RNA Structure, Interactions and Folding, Pt B. 2009;469:237–251. - PubMed
-
- Baird NJ, West J, Sosnick TR. RNA Structure and Folding Analyzed Using Small-Angle X-Ray Scattering. Handbook of RNA Biochemistry, Vols 1 and 2, 2nd Edition. 2014:407–425.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

