Inflammatory properties of inhibitor of DNA binding 1 secreted by synovial fibroblasts in rheumatoid arthritis
- PMID: 27071670
- PMCID: PMC4830090
- DOI: 10.1186/s13075-016-0984-3
Inflammatory properties of inhibitor of DNA binding 1 secreted by synovial fibroblasts in rheumatoid arthritis
Abstract
Background: Inhibitor of DNA binding 1 (Id1) is a nuclear protein containing a basic helix-loop-helix (bHLH) domain that regulates cell growth by selective binding and prevention of gene transcription. Sources of Id1 production in rheumatoid arthritis synovial tissue (RA ST) and its range of functional effects in RA remain to be clarified.
Methods: We analyzed Id1 produced from synovial fibroblasts and endothelial cells (ECs) with histology and real-time polymerase chain reaction (RT-PCR). Fibroblast supernatants subjected to differential centrifugation to isolate and purify exosomes were measured for Id1 by enzyme-linked immunosorbent assay (ELISA). Western blotting of Id1-stimulated ECs was performed to determine the kinetics of intracellular protein phosphorylation. EC intracellular signaling pathways induced by Id1 were subsequently targeted with silencing RNA (siRNA) for angiogenesis inhibition.
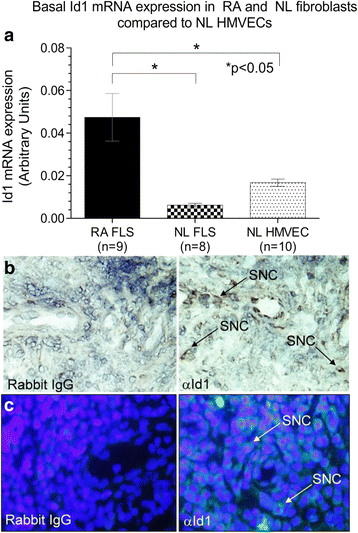
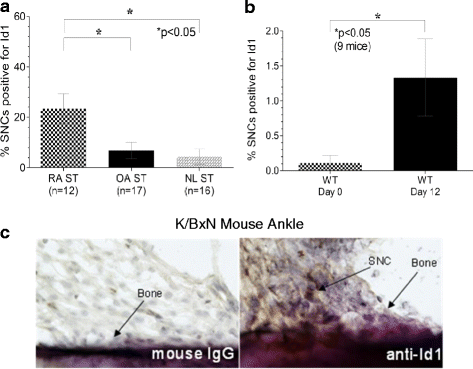
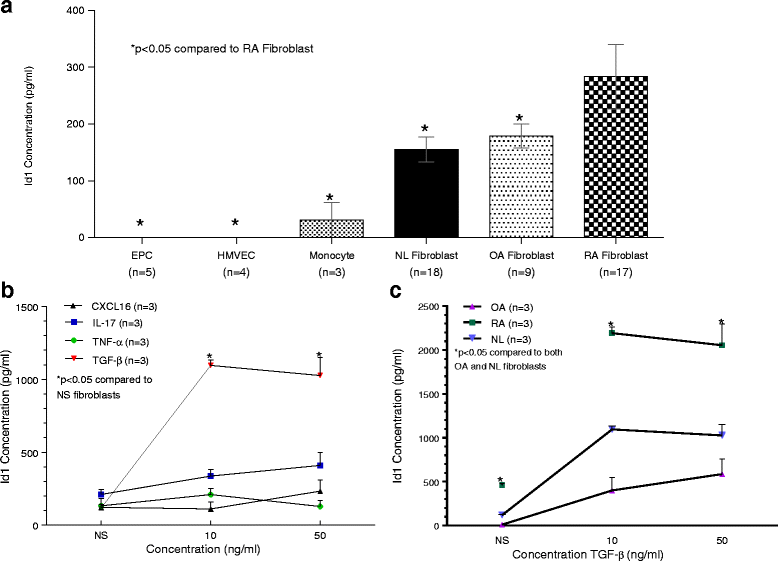
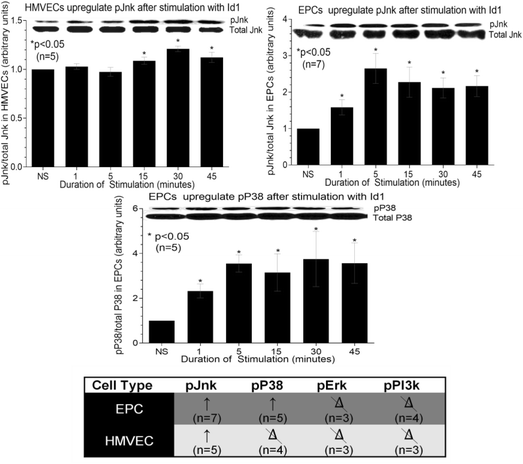
Results: By PCR and histologic analysis, we found that the primary source of Id1 in STs is from activated fibroblasts that correlate with inflammatory scores in human RA ST and in joints from K/BxN serum-induced mice. Normal (NL) and RA synovial fibroblasts increase Id1 production with stimulation by transforming growth factor beta (TGF-β). Most of the Id1 released by RA synovial fibroblasts is contained within exosomes. Endothelial progenitor cells (EPCs) and human dermal microvascular ECs (HMVECs) activate the Jnk signaling pathway in response to Id1, and Jnk siRNA reverses Id1-induced HMVEC vessel formation in Matrigel plugs in vivo.
Conclusions: Id1 is a pleotropic molecule affecting angiogenesis, vasculogenesis, and fibrosis. Our data shows that Id1 is not only an important nuclear protein, but also can be released from fibroblasts via exosomes. The ability of extracellular Id1 to activate signaling pathways expands the role of Id1 in the orchestration of tissue inflammation.
Keywords: Angiogenesis; Fibroblasts; Inflammation; Inhibitor of DNA binding-1 protein (Id1); Rheumatoid arthritis.
Figures


References
-
- Mellick AS, Plummer PN, Nolan DJ, Gao D, Bambino K, Hahn M, et al. Using the transcription factor inhibitor of DNA binding 1 to selectively target endothelial progenitor cells offers novel strategies to inhibit tumor angiogenesis and growth. Cancer Res. 2010;70(18):7273–7282. doi: 10.1158/0008-5472.CAN-10-1142. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

