Improving fascin inhibitors to block tumor cell migration and metastasis
- PMID: 27071719
- PMCID: PMC5423182
- DOI: 10.1016/j.molonc.2016.03.006
Improving fascin inhibitors to block tumor cell migration and metastasis
Abstract
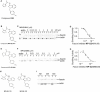
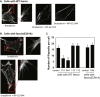
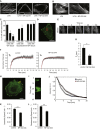
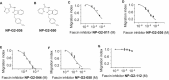
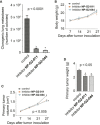
Tumor metastasis is the major cause of mortality of cancer patients, being responsible for ∼90% of all cancer deaths. One of the key steps during tumor metastasis is tumor cell migration which requires actin cytoskeletal reorganization. Among the critical actin cytoskeletal protrusion structures are antenna-like filopodia. Fascin protein is the main actin-bundling protein in filopodia. Here we report the development of fascin-specific small-molecules that inhibit the interaction between fascin and actin. These inhibitors block the in vitro actin-binding and actin-bundling activities of fascin, tumor cell migration and tumor metastasis in mouse models. Mechanistically, these inhibitors likely occupy one of the actin-binding sites, reduce the binding of actin filaments, and thus lead to the inhibition of the bundling activity of fascin. At the cellular level, these inhibitors impair actin cytoskeletal reorganization. Our data indicate that target-specific anti-fascin agents will have great potential for treating metastatic tumors.
Keywords: Fascin; Inhibitors; Metastasis.
Copyright © 2016 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Targeted inhibition of fascin function blocks tumour invasion and metastatic colonization.Nat Commun. 2015 Jun 17;6:7465. doi: 10.1038/ncomms8465. Nat Commun. 2015. PMID: 26081695
-
Structural Insights into the Induced-fit Inhibition of Fascin by a Small-Molecule Inhibitor.J Mol Biol. 2018 Apr 27;430(9):1324-1335. doi: 10.1016/j.jmb.2018.03.009. Epub 2018 Mar 21. J Mol Biol. 2018. PMID: 29573988 Free PMC article.
-
Mechanism of actin filament bundling by fascin.J Biol Chem. 2011 Aug 26;286(34):30087-96. doi: 10.1074/jbc.M111.251439. Epub 2011 Jun 18. J Biol Chem. 2011. PMID: 21685497 Free PMC article.
-
The post-translational modification of Fascin: impact on cell biology and its associations with inhibiting tumor metastasis.Amino Acids. 2022 Dec;54(12):1541-1552. doi: 10.1007/s00726-022-03193-x. Epub 2022 Aug 8. Amino Acids. 2022. PMID: 35939077 Review.
-
How does fascin promote cancer metastasis?FEBS J. 2021 Mar;288(5):1434-1446. doi: 10.1111/febs.15484. Epub 2020 Jul 23. FEBS J. 2021. PMID: 32657526 Free PMC article. Review.
Cited by
-
Quantitative assessment of cell contractility using polarized light microscopy.J Biophotonics. 2018 Nov;11(11):e201800008. doi: 10.1002/jbio.201800008. Epub 2018 Jul 11. J Biophotonics. 2018. PMID: 29931742 Free PMC article.
-
Novel anti-invasive properties of a Fascin1 inhibitor on colorectal cancer cells.J Mol Med (Berl). 2020 Mar;98(3):383-394. doi: 10.1007/s00109-020-01877-z. Epub 2020 Jan 29. J Mol Med (Berl). 2020. PMID: 31996952
-
Fascin inhibitor increases intratumoral dendritic cell activation and anti-cancer immunity.Cell Rep. 2021 Apr 6;35(1):108948. doi: 10.1016/j.celrep.2021.108948. Cell Rep. 2021. PMID: 33826900 Free PMC article.
-
Fascin-1 and its role as a serological marker in prostate cancer: a prospective case-control study.Future Sci OA. 2021 Jun 30;7(9):FSO745. doi: 10.2144/fsoa-2021-0051. eCollection 2021 Oct. Future Sci OA. 2021. PMID: 34737886 Free PMC article.
-
Ubiquitin ligase TRIM65 promotes colorectal cancer metastasis by targeting ARHGAP35 for protein degradation.Oncogene. 2019 Sep;38(37):6429-6444. doi: 10.1038/s41388-019-0891-6. Epub 2019 Jul 22. Oncogene. 2019. PMID: 31332286 Free PMC article.
References
-
- Adams, J.C. , 2004. Roles of fascin in cell adhesion and motility. Curr. Opin. Cell Biol. 16, 590–596. - PubMed
-
- Aznavoorian, S. , Murphy, A.N. , Stetler-Stevenson, W.G. , Liotta, L.A. , 1993. Molecular aspects of tumor cell invasion and metastasis. Cancer. 71, 1368–1383. - PubMed
-
- Bentley, D. , Toroian-Raymond, A. , 1986. Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature. 323, 712–715. - PubMed
-
- Bryan, J. , Kane, R.E. , 1978. Separation and interaction of the major components of sea urchin actin gel. J. Mol. Biol. 125, 207–224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

