Impact of Disease Duration on the Effects of Pramlintide in Type 1 Diabetes: A Post Hoc Analysis of Three Clinical Trials
- PMID: 27071768
- PMCID: PMC4882374
- DOI: 10.1007/s12325-016-0326-5
Impact of Disease Duration on the Effects of Pramlintide in Type 1 Diabetes: A Post Hoc Analysis of Three Clinical Trials
Abstract
Introduction: Adjunctive mealtime use of the amylin analog pramlintide improves postprandial hyperglycemia in patients with type 1 diabetes. This post hoc analysis of three randomized trials evaluated whether disease duration affected responses to pramlintide.
Methods: Patients received mealtime pramlintide 30 or 60 µg (n = 714) or placebo (n = 537) as an adjunct to insulin and were stratified into tertiles by diabetes duration at baseline. Efficacy and safety end points were assessed at week 26 using analysis of covariance and logistic regression models.
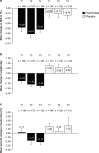
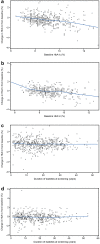
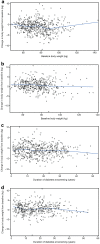
Results: Disease durations for tertiles 1, 2, and 3 were 6.7, 16.5, and 29.9 years, respectively. In all tertiles, pramlintide resulted in greater reductions in glycated hemoglobin (HbA1c) and weight than placebo, with greater weight reductions and insulin sparing in tertiles 2 and 3. Insulin dose and weight increased in the placebo group in all tertiles. Baseline HbA1c was a predictor of HbA1c lowering in both treatment groups (P < 0.0001); higher daily insulin predicted a smaller percent increase in insulin dose for placebo (P = 0.01); and higher body weight predicted greater weight loss in both pramlintide- and placebo-treated patients (P < 0.05). Event rates for severe hypoglycemia were similar for pramlintide and placebo and increased with longer duration of diabetes for both groups. Nausea with pramlintide increased with longer disease duration.
Conclusion: Mealtime pramlintide resulted in greater reductions in HbA1c than placebo, regardless of diabetes duration at baseline. Longer disease duration appeared to augment insulin sparing and weight loss with pramlintide, with a potential for increased incidence of hypoglycemia and nausea.
Funding: The design and conduct of the study were supported by Amylin Pharmaceuticals, San Diego, CA, USA.
Keywords: Disease duration; Efficacy; Endocrinology; Insulin; Pramlintide; Type 1 diabetes.
Figures
Similar articles
-
Pramlintide improved measures of glycemic control and body weight in patients with type 1 diabetes mellitus undergoing continuous subcutaneous insulin infusion therapy.Postgrad Med. 2013 May;125(3):136-44. doi: 10.3810/pgm.2013.05.2635. Postgrad Med. 2013. PMID: 23748514 Clinical Trial.
-
Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial.Diabetes Care. 2003 Mar;26(3):784-90. doi: 10.2337/diacare.26.3.784. Diabetes Care. 2003. PMID: 12610038 Clinical Trial.
-
Adjunctive therapy with the amylin analogue pramlintide leads to a combined improvement in glycemic and weight control in insulin-treated subjects with type 2 diabetes.Diabetes Technol Ther. 2002;4(1):51-61. doi: 10.1089/15209150252924094. Diabetes Technol Ther. 2002. PMID: 12017421 Clinical Trial.
-
Pramlintide acetate injection for the treatment of type 1 and type 2 diabetes mellitus.Clin Ther. 2007 Apr;29(4):535-62. doi: 10.1016/j.clinthera.2007.04.005. Clin Ther. 2007. PMID: 17617279 Review.
-
Efficacy and harms of the hypoglycemic agent pramlintide in diabetes mellitus.Ann Fam Med. 2010 Nov-Dec;8(6):542-9. doi: 10.1370/afm.1174. Ann Fam Med. 2010. PMID: 21060125 Free PMC article. Review.
Cited by
-
Pharmacological Treatment in Diabetes Mellitus Type 1 - Insulin and What Else?Int J Endocrinol Metab. 2017 Nov 20;16(1):e13008. doi: 10.5812/ijem.13008. eCollection 2018 Jan. Int J Endocrinol Metab. 2017. PMID: 29696037 Free PMC article. Review.
-
Efficacy and safety of pramlintide injection adjunct to insulin therapy in patients with type 1 diabetes mellitus: a systematic review and meta-analysis.Oncotarget. 2017 Mar 8;8(39):66504-66515. doi: 10.18632/oncotarget.16008. eCollection 2017 Sep 12. Oncotarget. 2017. PMID: 29029531 Free PMC article.
-
Structural insight into selectivity of amylin and calcitonin receptor agonists.Nat Chem Biol. 2024 Feb;20(2):162-169. doi: 10.1038/s41589-023-01393-4. Epub 2023 Aug 3. Nat Chem Biol. 2024. PMID: 37537379
-
Weight Management in Patients with Type 1 Diabetes and Obesity.Curr Diab Rep. 2017 Aug 23;17(10):92. doi: 10.1007/s11892-017-0918-8. Curr Diab Rep. 2017. PMID: 28836234 Free PMC article. Review.
References
-
- Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

