An optimized staining technique for the detection of Gram positive and Gram negative bacteria within tissue
- PMID: 27071769
- PMCID: PMC4828829
- DOI: 10.1186/s13104-016-1902-0
An optimized staining technique for the detection of Gram positive and Gram negative bacteria within tissue
Abstract
Background: Bacterial infections are a common clinical problem in both acute and chronic wounds. With growing concerns over antibiotic resistance, treatment of bacterial infections should only occur after positive diagnosis. Currently, diagnosis is delayed due to lengthy culturing methods which may also fail to identify the presence of bacteria. While newer costly bacterial identification methods are being explored, a simple and inexpensive diagnostic tool would aid in immediate and accurate treatments for bacterial infections. Histologically, hematoxylin and eosin (H&E) and Gram stains have been employed, but are far from optimal when analyzing tissue samples due to non-specific staining. The goal of the current study was to develop a modification of the Gram stain that enhances the contrast between bacteria and host tissue.
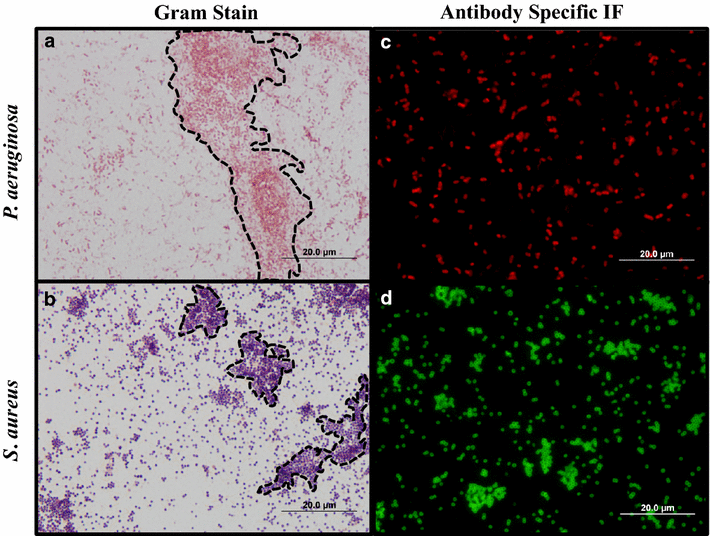
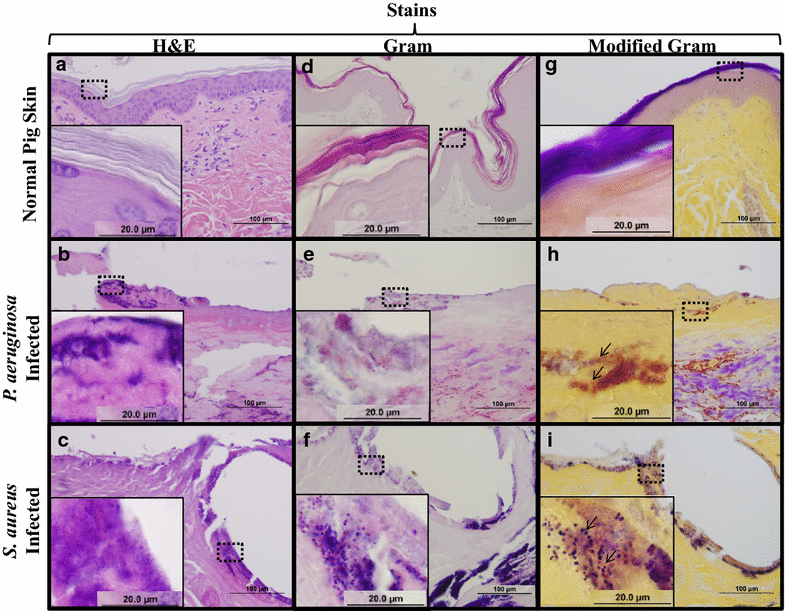
Findings: A modified Gram stain was developed and tested as an alternative to Gram stain that improves the contrast between Gram positive bacteria, Gram negative bacteria and host tissue. Initially, clinically relevant strains of Pseudomonas aeruginosa and Staphylococcus aureus were visualized in vitro and in biopsies of infected, porcine burns using routine Gram stain, and immunohistochemistry techniques involving bacterial strain-specific fluorescent antibodies as validation tools. H&E and Gram stain of serial biopsy sections were then compared to a modification of the Gram stain incorporating a counterstain that highlights collagen found in tissue. The modified Gram stain clearly identified both Gram positive and Gram negative bacteria, and when compared to H&E or Gram stain alone provided excellent contrast between bacteria and non-viable burn eschar. Moreover, when applied to surgical biopsies from patients that underwent burn debridement this technique was able to clearly detect bacterial morphology within host tissue.
Conclusions: We describe a modification of the Gram stain that provides improved contrast of Gram positive and Gram negative microorganisms within host tissue. The samples used in this study demonstrate that this staining technique has laboratory and clinical applicability. This modification only adds minutes to traditional Gram stain with reusable reagents, and results in a cost- and time-efficient technique for identifying bacteria in any clinical biopsy containing connective tissue.
Keywords: Burn; Gram’s stain; Histology; Infection; Pseudomonas aeruginosa; Staphylococcus aureus.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

