A novel open-porous magnesium scaffold with controllable microstructures and properties for bone regeneration
- PMID: 27071777
- PMCID: PMC4829853
- DOI: 10.1038/srep24134
A novel open-porous magnesium scaffold with controllable microstructures and properties for bone regeneration
Abstract
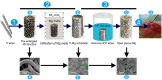
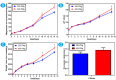
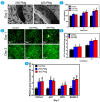
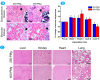
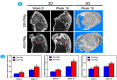
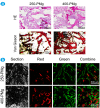
The traditional production methods of porous magnesium scaffolds are difficult to accurately control the pore morphologies and simultaneously obtain appropriate mechanical properties. In this work, two open-porous magnesium scaffolds with different pore size but in the nearly same porosity are successfully fabricated with high-purity Mg ingots through the titanium wire space holder (TWSH) method. The porosity and pore size can be easily, precisely and individually controlled, as well as the mechanical properties also can be regulated to be within the range of human cancellous bone by changing the orientation of pores without sacrifice the requisite porous structures. In vitro cell tests indicate that the scaffolds have good cytocompatibility and osteoblastic differentiation properties. In vivo findings demonstrate that both scaffolds exhibit acceptable inflammatory responses and can be almost fully degraded and replaced by newly formed bone. More importantly, under the same porosity, the scaffolds with larger pore size can promote early vascularization and up-regulate collagen type 1 and OPN expression, leading to higher bone mass and more mature bone formation. In conclusion, a new method is introduced to develop an open-porous magnesium scaffold with controllable microstructures and mechanical properties, which has great potential clinical application for bone reconstruction in the future.
Figures


Similar articles
-
Effect of the biodegradation rate controlled by pore structures in magnesium phosphate ceramic scaffolds on bone tissue regeneration in vivo.Acta Biomater. 2016 Oct 15;44:155-67. doi: 10.1016/j.actbio.2016.08.039. Epub 2016 Aug 21. Acta Biomater. 2016. PMID: 27554019
-
Effect of collagen-glycosaminoglycan scaffold pore size on matrix mineralization and cellular behavior in different cell types.J Biomed Mater Res A. 2016 Jan;104(1):291-304. doi: 10.1002/jbm.a.35567. Epub 2015 Oct 1. J Biomed Mater Res A. 2016. PMID: 26386362
-
Additively manufactured biodegradable porous iron.Acta Biomater. 2018 Sep 1;77:380-393. doi: 10.1016/j.actbio.2018.07.011. Epub 2018 Jul 6. Acta Biomater. 2018. PMID: 29981948
-
Role of pore size and morphology in musculo-skeletal tissue regeneration.Mater Sci Eng C Mater Biol Appl. 2016 Apr 1;61:922-39. doi: 10.1016/j.msec.2015.12.087. Epub 2015 Dec 31. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 26838923 Review.
-
Porous Scaffolds for Regeneration of Cartilage, Bone and Osteochondral Tissue.Adv Exp Med Biol. 2018;1058:171-191. doi: 10.1007/978-3-319-76711-6_8. Adv Exp Med Biol. 2018. PMID: 29691822 Review.
Cited by
-
In vivo gene delivery mediated by non-viral vectors for cancer therapy.J Control Release. 2020 Sep 10;325:249-275. doi: 10.1016/j.jconrel.2020.06.038. Epub 2020 Jul 4. J Control Release. 2020. PMID: 32634464 Free PMC article. Review.
-
Mimicking the Hierarchical Organization of Natural Collagen: Toward the Development of Ideal Scaffolding Material for Tissue Regeneration.Front Bioeng Biotechnol. 2021 Apr 27;9:644595. doi: 10.3389/fbioe.2021.644595. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33987173 Free PMC article. Review.
-
Three-dimensionally printed biphasic calcium phosphate blocks with different pore diameters for regeneration in rabbit calvarial defects.Biomater Res. 2022 Jun 15;26(1):25. doi: 10.1186/s40824-022-00271-9. Biomater Res. 2022. PMID: 35706067 Free PMC article.
-
Characterization of non-solvent- and thermal-induced phase separation applied polycaprolactone/demineralized bone matrix scaffold for bone tissue engineering.In Vitro Model. 2022 Apr 26;1(2):197-207. doi: 10.1007/s44164-022-00018-9. eCollection 2022 Apr. In Vitro Model. 2022. PMID: 39872803 Free PMC article.
-
Composite Polylactide/Polycaprolactone Foams with Hierarchical Porous Structure for Pre-Vascularized Tissue Engineering.Int J Mol Sci. 2025 Mar 25;26(7):2974. doi: 10.3390/ijms26072974. Int J Mol Sci. 2025. PMID: 40243624 Free PMC article.
References
-
- Wu S. L., Liu X. M., Yeung K. W. K., Liu C. S. & Yang X. J. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R 80, 1–36 (2014).
-
- Bauer T. W. & Muschler G. F. Bone graft materials-An overview of the basic science. Clin. Orthop. Relat. Res. 371, 10–27 (2000). - PubMed
-
- Greenwald A. S. et al. Bone-graft substitutes: Facts, fictions, and applications. J. Bone Jt. Surg. Am. Vol.83A, 98–103 (2001). - PubMed
-
- Lalk M. et al. Fluoride and calcium-phosphate coated sponges of the magnesium alloy AX30 as bone grafts: a comparative study in rabbits. J. Mater. Sci. Mater. Med. 24, 417–436 (2013). - PubMed
-
- Nandi S. K. et al. Orthopaedic applications of bone graft & graft substitutes: a review. Indian J. Med. Res. 132, 15–30 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

