Comparison of intravenous and subcutaneous exposure supporting dose selection of subcutaneous belimumab systemic lupus erythematosus Phase 3 program
- PMID: 27072354
- PMCID: PMC5054300
- DOI: 10.1177/0961203316642309
Comparison of intravenous and subcutaneous exposure supporting dose selection of subcutaneous belimumab systemic lupus erythematosus Phase 3 program
Abstract
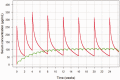
Background Belimumab is a recombinant, human, IgG1λ monoclonal antibody that targets B-lymphocyte stimulator. The intravenous formulation is indicated for the treatment of active, autoantibody-positive systemic lupus erythematosus (SLE). Belimumab has been formulated for subcutaneous (SC) administration to improve patient convenience. This post-hoc modeling and simulation analysis characterizes the population pharmacokinetics (PK) of SC belimumab, and compares the exposure profiles of the approved belimumab IV dose-10 mg/kg every four weeks-to the 200 mg SC weekly dose in SLE patients, highlighting key pharmacological differences relevant for clinicians. Methods Data from two Phase 1 studies in US American and Japanese healthy subjects were analyzed with a non-linear mixed effects modeling approach. The resulting SC population PK model and a previously developed IV population PK model were used to conduct simulation trials in a Phase 3 IV belimumab SLE patient population, comparing chronic exposure profiles and exposure ranges stratified by body weight tertiles for IV vs SC dosing. Results The PK of belimumab following SC administration was best described by a linear two-comment model. The estimates for clearance, steady-state volume of distribution, and bioavailability were 208 mL/day, 5250 mL, and 76%, respectively. After four weeks of SC dosing, simulated belimumab concentrations exceeded the steady-state trough concentrations of the IV dosing regimen. At steady state simulated serum profiles demonstrated comparable average belimumab concentrations (Cavg,ss) after IV and SC dosing. Simulated belimumab exposures demonstrated largely overlapping concentration ranges following 200 mg SC weekly and 10 mg/kg IV every four weeks dosing. Discussion The predicted Cavg,ss of belimumab in SLE patients was comparable following 200 mg SC weekly and 10 mg/kg IV every four weeks dosing. The simulated belimumab accumulation following SC weekly dosing indicated that administration of a loading dose was not required. Similar Cavg,ss ranges were predicted for fixed dose SC and weight-proportional IV regimens in the simulated SLE population, albeit with a reversed body-size-to-exposure relationship for the SC regimen. These findings provide rheumatologists with a better understanding of expected differences in belimumab exposure when comparing IV and SC dosing regimens.
Keywords: Systemic lupus erythematosus; belimumab; intravenous; pharmacokinetics; subcutaneous.
Figures


References
-
- Petri M, Stohl W, Chatham W, et al. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum 2008; 58: 2453–2459. - PubMed
-
- Zhang J, Roschke V, Baker KP, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol 2001; 166: 6–10. - PubMed
-
- Halpern WG, Lappin P, Zanardi T, et al. Chronic administration of belimumab, a BLyS antagonist decreases tissue and peripheral blood B-lymphocyte populations in Cynomolgus Monkeys: pharmacokinetic, pharmacodynamic, and toxicologic effects. Toxicol Sci 2006; 91: 586–599. - PubMed
-
- Manzi S, Sánchez-Guerrero J, Merrill JT, et al. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Ann Rheum Dis 2012; 71: 1833–1838. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

