Omega-3 LCPUFA supplement: a nutritional strategy to prevent maternal and neonatal oxidative stress
- PMID: 27072591
- PMCID: PMC6865966
- DOI: 10.1111/mcn.12300
Omega-3 LCPUFA supplement: a nutritional strategy to prevent maternal and neonatal oxidative stress
Abstract
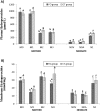
There is controversy about fish-oil supplementation and oxidative damage. This ambiguity should be explored to elucidate its role as modulator of oxidative stress, especially during gestation and postnatal life. This is the objective of this study. One hundred ten pregnant women were divided in two groups: control group CT (400 mL/day of the control dairy drink); supplemented group FO (400 mL/day of the fish oil-enriched dairy drink (±400-mg EPA-DHA/day)). Different biomarkers of oxidative damage were determined in the mother's at enrolment, at delivery and at 2.5 and 4 months postpartum and newborns at delivery and at 2.5 months postpartum. Omega-3 LC-PUFA supplementation during pregnancy and lactation decreased plasma hydroperoxides especially in newborn at delivery (P = 0.001) and 2.5 months (P = 0.006), increased superoxide dismutase (SOD) and catalase (CAT) in mothers at delivery (P = 0.024 (SOD)) and after 2.5 months (P = 0.040 (CAT)) and in newborns at 2.5 months (P = 0.035 (SOD); P = 0.021 (CAT)). Also, supplementation increased α-tocoferol in mothers at 2.5 months (P = 0.030) and in umbilical cord artery (P = 0.039). Higher levels of CoQ10 were found in mothers at delivery (P = 0.039) as well as in umbilical cord vein (P = 0.024) and artery (P = 0.036). Our supplementation prevents the oxidative stress in the mother and neonate during the first months of postnatal life, being a potential preventive nutritional strategy to prevent functional alterations associated with oxidative stress that have an important repercussion for the neonate development in the early postnatal life.
Keywords: antioxidant defence; docosahexaenoic acid (DHA); infant development; n-3 fatty acids; oxidative stress; pregnancy.
© 2016 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
-
- Barden A.E., Mori T.A., Dunstan J.A., Taylor A.L., Thornton C.A., Croft K.D. et al. (2004) Fish oil supplementation in pregnancy lowers F2‐isoprostanes in neonates at high risk of atopy. Free Radical Research 38, 233–239. - PubMed
-
- Boulis T.S., Rochelson B., Novick O., Xue X., Chatterjee P.K., Gupta M. et al. (2014) Omega‐3 polyunsaturated fatty acids enhance cytokine production and oxidative stress in a mouse model of preterm labor. Journal of Perinatal Medicine 42, 693–698. - PubMed
-
- Bouzidi N., Mekki K., Boukaddoum A., Dida N., Kaddous A. & Bouchenak M. (2010) Effects of omega‐3 poly unsaturated fatty‐acid supplementation on redox status in chronic renal failure patients with dyslipidemia. Journal of Renal Nutrition 20, 321–328. - PubMed
-
- Cai F., Sorg O., Granci V., Lecumberri E., Miralbell R., Dupertuis Y.M. et al. (2014) Interaction of ω‐3 polyunsaturated fatty acids with radiation therapy in two different colorectal cancer cell lines. Clinical Nutrition 33, 164–170. - PubMed
-
- Campoy C., Escolano‐Margarit M.V., Anjos T., Szajewska H. & Uauy R. (2012) Omega 3 fatty acids on child growth, visual acuity and neurodevelopment. British Journal of Nutrition 107, S85–S106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

