DNA hypomethylation upregulates expression of the MGAT3 gene in HepG2 cells and leads to changes in N-glycosylation of secreted glycoproteins
- PMID: 27073020
- PMCID: PMC4829869
- DOI: 10.1038/srep24363
DNA hypomethylation upregulates expression of the MGAT3 gene in HepG2 cells and leads to changes in N-glycosylation of secreted glycoproteins
Abstract
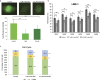
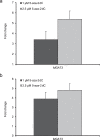
Changes in N-glycosylation of plasma proteins are observed in many types of cancer, nevertheless, few studies suggest the exact mechanism involved in aberrant protein glycosylation. Here we studied the impact of DNA methylation on the N-glycome in the secretome of the HepG2 cell line derived from hepatocellular carcinoma (HCC). Since the majority of plasma glycoproteins originate from the liver, the HepG2 cells represent a good model for glycosylation changes in HCC that are detectable in blood, which is an easily accessible analytic material in a clinical setting. Two different concentrations of 5-aza-2'-deoxycytidine (5-aza-2dC) differentially affected global genome methylation and induced different glycan changes. Around twenty percent of 84 glyco-genes analysed changed expression level after the 5-aza-2dC treatment as a result of global genome hypomethylation. A correlation study between the changes in glyco-gene expression and the HepG2 glycosylation profile suggests that the MGAT3 gene might be responsible for the glycan changes consistently induced by both doses of 5-aza-2dC. Core-fucosylated tetra-antennary structures were decreased in quantity likely as a result of hypomethylated MGAT3 gene promoter followed by increased expression of this gene.
Figures





References
-
- Dalziel M., Crispin M., Scanlan C. N., Zitzmann N. & Dwek R. A. Emerging principles for the therapeutic exploitation of glycosylation. Science 3343, 1235681-1–1235681-8 (2014). - PubMed
-
- Zoldoš V., Novokmet M., Bečeheli I. & Lauc G. Genomics and epigenomics of human glycome. Glycoconj. J. 30, 41–50 (2013). - PubMed
-
- Krištić J., Zoldoš V. & Lauc G. Complex genetics of protein glycosylation In Glycoscience: Biology and Medicine (eds Taniguchi N., Endo T., Hart G. W., Seeberger P. H. & Wong C. H.) Ch. 8, 1303–1310 (Springer Japan, 2015).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

