Organotypic slice cultures of human gastric and esophagogastric junction cancer
- PMID: 27073068
- PMCID: PMC4944870
- DOI: 10.1002/cam4.720
Organotypic slice cultures of human gastric and esophagogastric junction cancer
Abstract
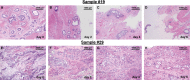
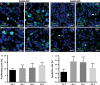
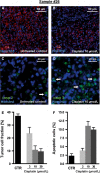
Gastric and esophagogastric junction cancers are heterogeneous and aggressive tumors with an unpredictable response to cytotoxic treatment. New methods allowing for the analysis of drug resistance are needed. Here, we describe a novel technique by which human tumor specimens can be cultured ex vivo, preserving parts of the natural cancer microenvironment. Using a tissue chopper, fresh surgical tissue samples were cut in 400 μm slices and cultivated in 6-well plates for up to 6 days. The slices were processed for routine histopathology and immunohistochemistry. Cytokeratin stains (CK8, AE1/3) were applied for determining tumor cellularity, Ki-67 for proliferation, and cleaved caspase-3 staining for apoptosis. The slices were analyzed under naive conditions and following 2-4 days in vitro exposure to 5-FU and cisplatin. The slice culture technology allowed for a good preservation of tissue morphology and tumor cell integrity during the culture period. After chemotherapy exposure, a loss of tumor cellularity and an increase in apoptosis were observed. Drug sensitivity of the tumors could be assessed. Organotypic slice cultures of gastric and esophagogastric junction cancers were successfully established. Cytotoxic drug effects could be monitored. They may be used to examine mechanisms of drug resistance in human tissue and may provide a unique and powerful ex vivo platform for the prediction of treatment response.
Keywords: Chemosensitivity; esophagogastric junction cancer; gastric cancer; organotypic slice cultures; personalized treatment.
© 2016 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures



References
-
- Lordick, F. , Lorenzen S., Yamada Y., and Ilson D.. 2014. Optimal chemotherapy for advanced gastric cancer: is there a global consensus? Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 17:213–225. - PubMed
-
- Cunningham, D. , Allum W. H., Stenning S. P., Thompson J. N., Van de Velde C. J. H., Nicolson M., et al. 2006. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 355:11–20. - PubMed
-
- Ychou, M. , Boige V., Pignon J.‐P., Conroy T., Bouché O., Lebreton G., et al. 2011. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 29:1715–1721. - PubMed
-
- Van Hagen, P. , Hulshof M. C. C. M., van Lanschot J. J. B., Steyerberg E. W., van Berge Henegouwen M. I., Wijnhoven B. P. L., et al. 2012. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 366:2074–2084. - PubMed
-
- Smalley, S. R. , Benedetti J. K., Haller D. G., Hundahl S. A., Estes N. C., Ajani J. A., et al. 2012. Updated analysis of SWOG‐directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 30:2327–2333. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

