Cytosolic Replication of Group A Streptococcus in Human Macrophages
- PMID: 27073088
- PMCID: PMC4959517
- DOI: 10.1128/mBio.00020-16
Cytosolic Replication of Group A Streptococcus in Human Macrophages
Erratum in
-
Erratum for O'Neill et al., Cytosolic Replication of Group A Streptococcus in Human Macrophages.mBio. 2016 Jun 28;7(3):e00931-16. doi: 10.1128/mBio.00931-16. mBio. 2016. PMID: 27353761 Free PMC article. No abstract available.
Abstract
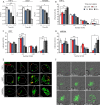
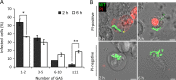
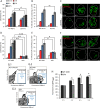
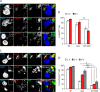
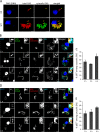
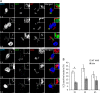
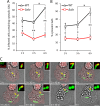
As key components of innate immune defense, macrophages are essential in controlling bacterial pathogens, including group A Streptococcus(GAS). Despite this, only a limited number of studies have analyzed the recovery of GAS from within human neutrophils and macrophages. Here, we determined the intracellular fate of GAS in human macrophages by using several quantitative approaches. In both U937 and primary human macrophages, the appearance over time of long GAS chains revealed that despite GAS-mediated cytotoxicity, replication occurred in viable, propidium iodide-negative macrophages. Whereas the major virulence factor M1 did not contribute to bacterial growth, a GAS mutant strain deficient in streptolysin O (SLO) was impaired for intracellular replication. SLO promoted bacterial escape from the GAS-containing vacuole (GCV) into the macrophage cytosol. Up to half of the cytosolic GAS colocalized with ubiquitin and p62, suggesting that the bacteria were targeted by the autophagy machinery. Despite this, live imaging of U937 macrophages revealed proficient replication of GAS after GCV rupture, indicating that escape from the GCV is important for growth of GAS in macrophages. Our results reveal that GAS can replicate within viable human macrophages, with SLO promoting GCV escape and cytosolic growth, despite the recruitment of autophagy receptors to bacteria.
Importance: Classically regarded as an extracellular pathogen, GAS can persist within human epithelial cells, as well as neutrophils and macrophages. Some studies suggest that GAS can modulate its intracellular vacuole to promote survival and perhaps replicate in macrophages. However, an in-depth single-cell analysis of the dynamics of survival and replication is lacking. We used macrophage-like cell lines and primary macrophages to measure the intracellular growth of GAS at both the population and single-cell levels. While CFU counts revealed no increase in overall bacterial growth, quantitative fluorescence microscopy, flow cytometry, and time-lapse imaging revealed bacterial replication in a proportion of infected macrophages. This study emphasizes the importance of single-cell analysis especially when studying the intracellular fate of a pathogen that is cytotoxic and displays heterogeneity in terms of intracellular killing and growth. To our knowledge, this study provides the first direct visualization of GAS replication inside human cells.
Copyright © 2016 O’Neill et al.
Figures
Comment in
-
One More Disguise in the Stealth Behavior of Streptococcus pyogenes.mBio. 2016 May 17;7(3):e00661-16. doi: 10.1128/mBio.00661-16. mBio. 2016. PMID: 27190219 Free PMC article.
References
-
- Sakurai A, Maruyama F, Funao J, Nozawa T, Aikawa C, Okahashi N, Shintani S, Hamada S, Ooshima T, Nakagawa I. 2010. Specific behavior of intracellular Streptococcus pyogenes that has undergone autophagic degradation is associated with bacterial streptolysin O and host small G proteins Rab5 and Rab7. J Biol Chem 285:22666–22675. doi:10.1074/jbc.M109.100131. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
