The Players: Cells Involved in Glomerular Disease
- PMID: 27073196
- PMCID: PMC5012495
- DOI: 10.2215/CJN.13791215
The Players: Cells Involved in Glomerular Disease
Abstract
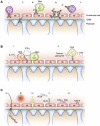
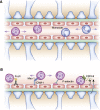
Glomerular diseases are common and important. They can arise from systemic inflammatory or metabolic diseases that affect the kidney. Alternately, they are caused primarily by local glomerular abnormalities, including genetic diseases. Both intrinsic glomerular cells and leukocytes are critical to the healthy glomerulus and to glomerular dysregulation in disease. Mesangial cells, endothelial cells, podocytes, and parietal epithelial cells within the glomerulus all play unique and specialized roles. Although a specific disease often primarily affects a particular cell type, the close proximity, and interdependent functions and interactions between cells mean that even diseases affecting one cell type usually indirectly influence others. In addition to those cells intrinsic to the glomerulus, leukocytes patrol the glomerulus in health and mediate injury in disease. Distinct leukocyte types and subsets are present, with some being involved in different ways in an individual glomerular disease. Cells of the innate and adaptive immune systems are important, directing systemic immune and inflammatory responses, locally mediating injury, and potentially dampening inflammation and facilitating repair. The advent of new genetic and molecular techniques, and new disease models means that we better understand both the basic biology of the glomerulus and the pathogenesis of glomerular disease. This understanding should lead to better diagnostic techniques, biomarkers, and predictors of prognosis, disease severity, and relapse. With this knowledge comes the promise of better therapies in the future, directed toward halting pathways of injury and fibrosis, or interrupting the underlying pathophysiology of the individual diseases that lead to significant and progressive glomerular disease.
Keywords: Glomerulus; Immunology and pathology; Inflammation; Leukocytes; Podocytes; Prognosis; endothelial cells; fibrosis; kidney; mesangial cells.
Copyright © 2016 by the American Society of Nephrology.
Figures


References
-
- Shankland SJ, Smeets B, Pippin JW, Moeller MJ: The emergence of the glomerular parietal epithelial cell. Nat Rev Nephrol 10: 158–173, 2014 - PubMed
-
- Schlöndorff D, Banas B: The mesangial cell revisited: no cell is an island. J Am Soc Nephrol 20: 1179–1187, 2009 - PubMed
-
- Saleem MA: One hundred ways to kill a podocyte. Nephrol Dial Transplant 30: 1266–1271, 2015 - PubMed
-
- Abboud HE: Mesangial cell biology. Exp Cell Res 318: 979–985, 2012 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

