Reduced Numbers and Impaired Function of Regulatory T Cells in Peripheral Blood of Ischemic Stroke Patients
- PMID: 27073295
- PMCID: PMC4814689
- DOI: 10.1155/2016/2974605
Reduced Numbers and Impaired Function of Regulatory T Cells in Peripheral Blood of Ischemic Stroke Patients
Abstract
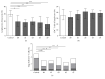
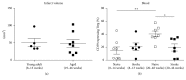
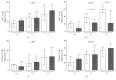
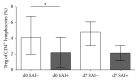
Background and purpose: Regulatory T cells (Tregs) have been suggested to modulate stroke-induced immune responses. However, analyses of Tregs in patients and in experimental stroke have yielded contradictory findings. We performed the current study to assess the regulation and function of Tregs in peripheral blood of stroke patients. Age dependent expression of CD39 on Tregs was quantified in mice and men.
Methods: Total FoxP3(+) Tregs and CD39(+)FoxP3(+) Tregs were quantified by flow cytometry in controls and stroke patients on admission and on days 1, 3, 5, and 7 thereafter. Treg function was assessed by quantifying the inhibition of activation-induced expression of CD69 and CD154 on T effector cells (Teffs).
Results: Total Tregs accounted for 5.0% of CD4(+) T cells in controls and <2.8% in stroke patients on admission. They remained below control values until day 7. CD39(+) Tregs were most strongly reduced in stroke patients. On day 3 the Treg-mediated inhibition of CD154 upregulation on CD4(+) Teff was impaired in stroke patients. CD39 expression on Treg increased with age in peripheral blood of mice and men.
Conclusion: We demonstrate a loss of active FoxP3(+)CD39(+) Tregs from stroke patient's peripheral blood. The suppressive Treg function of remaining Tregs is impaired after stroke.
Figures

References
-
- Prass K., Meisel C., Höflich C., et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1–like immunostimulation. The Journal of Experimental Medicine. 2003;198(5):725–736. doi: 10.1084/jem.20021098. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

