Serum CEACAM1 Level Is Associated with Diagnosis and Prognosis in Patients with Osteosarcoma
- PMID: 27074014
- PMCID: PMC4830595
- DOI: 10.1371/journal.pone.0153601
Serum CEACAM1 Level Is Associated with Diagnosis and Prognosis in Patients with Osteosarcoma
Abstract
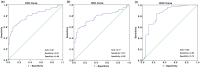
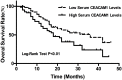
Carcinoembryonic antigen related cell adhesion molecule 1 (CEACAM1) is a trans-membrane multifunctional cell adhesion molecule associated with tumor cell proliferation, apoptosis, angiogenesis, invasion, and migration during tumor development. In the present study, we evaluated serum CEACAM1 level in osteosarcoma patients to explore its diagnostic and prognostic value for this particular malignancy. Sera from 113 patients with primary osteosarcoma, 98 patients with benign bone tumors and 126 healthy controls were obtained. Serum CEACAM1 level was measured with ELISA and correlation with clinicopathological characteristics was further analyzed. Receiver operating curves (ROC), Kaplan-Meier curves, and log-rank analyses as well as Cox proportional hazard models were used to evaluate diagnostic and prognostic significance. The results revealed that serum CEACAM1 level was significantly higher in osteosarcoma patients compared to benign bone tumors and healthy controls (455.2 ± 179.9 vs 287.4 ± 103.2, 260.8 ± 109.7 pg/ml, respectively). Osteosarcoma patients with larger tumors, later-tumor stages, low tumor grades, and distant metastases had much higher CEACAM1 compared to those with smaller tumors, earlier tumor stages, high tumor grades and non-distant metastases (P < 0.05 for all). Multivariate logistic regression analysis confirmed that high serum CEACAM1 level was an independent risk factor for distant metastases (OR = 3.02, 95%CI 1.65-4.17). To distinguish osteosarcoma patients from those with benign bone tumor and healthy controls, ROC/AUC analysis indicated an AUC of 0.81 (sensitivity 0.61; specificity 0.89) and an AUC of 0.77 (sensitivity 0.57; specificity 0.92), respectively. Osteosarcoma patients with higher CEACAM1 had relatively lower survival compared to those with low CEACAM1 (P < 0.01), and multivariate analyses for overall survival revealed that high serum CEACAM1 level was an independent prognostic factor for osteosarcoma (HR = 1.56, 95%CI 1.23-3.28). The present study suggested that elevated serum CEACAM1 level might be a novel diagnostic and prognostic biomarker for osteosarcoma patients.
Conflict of interest statement
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

