Notch Signaling and the Skeleton
- PMID: 27074349
- PMCID: PMC4890266
- DOI: 10.1210/er.2016-1002
Notch Signaling and the Skeleton
Abstract
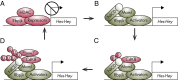
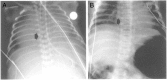
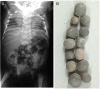
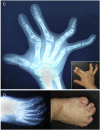
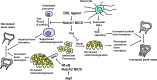
Notch 1 to 4 receptors are important determinants of cell fate and function, and Notch signaling plays an important role in skeletal development and bone remodeling. After direct interactions with ligands of the Jagged and Delta-like families, a series of cleavages release the Notch intracellular domain (NICD), which translocates to the nucleus where it induces transcription of Notch target genes. Classic gene targets of Notch are hairy and enhancer of split (Hes) and Hes-related with YRPW motif (Hey). In cells of the osteoblastic lineage, Notch activation inhibits cell differentiation and causes cancellous bone osteopenia because of impaired bone formation. In osteocytes, Notch1 has distinct effects that result in an inhibition of bone resorption secondary to an induction of osteoprotegerin and suppression of sclerostin with a consequent enhancement of Wnt signaling. Notch1 inhibits, whereas Notch2 enhances, osteoclastogenesis and bone resorption. Congenital disorders of loss- and gain-of-Notch function present with severe clinical manifestations, often affecting the skeleton. Enhanced Notch signaling is associated with osteosarcoma, and Notch can influence the invasive potential of carcinoma of the breast and prostate. Notch signaling can be controlled by the use of inhibitors of Notch activation, small peptides that interfere with the formation of a transcriptional complex, or antibodies to the extracellular domain of specific Notch receptors or to Notch ligands. In conclusion, Notch plays a critical role in skeletal development and homeostasis, and serious skeletal disorders can be attributed to alterations in Notch signaling.
Figures




References
-
- Lindsell CE, Boulter J, diSibio G, Gossler A, Weinmaster G. Expression patterns of Jagged, Delta1, Notch1, Notch2, and Notch3 genes identify ligand-receptor pairs that may function in neural development. Mol Cell Neurosci. 1996;8(1):14–27. - PubMed
-
- Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393(6683):382–386. - PubMed
-
- Kovall RA. More complicated than it looks: assembly of Notch pathway transcription complexes. Oncogene. 2008;27(38):5099–5109. - PubMed
-
- Visan I, Tan JB, Yuan JS, Harper JA, Koch U, Guidos CJ. Regulation of T lymphopoiesis by Notch1 and Lunatic fringe-mediated competition for intrathymic niches. Nat Immunol. 2006;7(6):634–643. - PubMed
-
- Dale JK, Maroto M, Dequeant ML, Malapert P, McGrew M, Pourquie O. Periodic notch inhibition by lunatic fringe underlies the chick segmentation clock. Nature. 2003;421(6920):275–278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

