Robust neuronal dynamics in premotor cortex during motor planning
- PMID: 27074502
- PMCID: PMC5081260
- DOI: 10.1038/nature17643
Robust neuronal dynamics in premotor cortex during motor planning
Erratum in
-
Corrigendum: Robust neuronal dynamics in premotor cortex during motor planning.Nature. 2016 Sep 1;537(7618):122. doi: 10.1038/nature18623. Epub 2016 Jun 29. Nature. 2016. PMID: 27362233 No abstract available.
Abstract
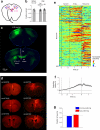
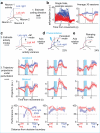
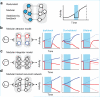
Neural activity maintains representations that bridge past and future events, often over many seconds. Network models can produce persistent and ramping activity, but the positive feedback that is critical for these slow dynamics can cause sensitivity to perturbations. Here we use electrophysiology and optogenetic perturbations in the mouse premotor cortex to probe the robustness of persistent neural representations during motor planning. We show that preparatory activity is remarkably robust to large-scale unilateral silencing: detailed neural dynamics that drive specific future movements were quickly and selectively restored by the network. Selectivity did not recover after bilateral silencing of the premotor cortex. Perturbations to one hemisphere are thus corrected by information from the other hemisphere. Corpus callosum bisections demonstrated that premotor cortex hemispheres can maintain preparatory activity independently. Redundancy across selectively coupled modules, as we observed in the premotor cortex, is a hallmark of robust control systems. Network models incorporating these principles show robustness that is consistent with data.
Figures


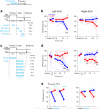
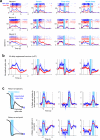



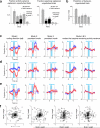
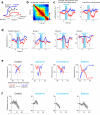




Comment in
-
Neuroscience: Fault tolerance in the brain.Nature. 2016 Apr 28;532(7600):449-50. doi: 10.1038/nature17886. Epub 2016 Apr 13. Nature. 2016. PMID: 27074510 No abstract available.
References
-
- Tanji J, Evarts EV. Anticipatory activity of motor cortex neurons in relation to direction of an intended movement. J Neurophysiol. 1976;39:1062–1068. - PubMed
-
- Murakami M, Vicente MI, Costa GM, Mainen ZF. Neural antecedents of self-initiated actions in secondary motor cortex. Nature neuroscience. 2014;17:1574–1582. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

